Unit-4
Heat treatment of Steel
Question 1: What is heat treatment? Define Annealing and Normalizing.
A1: Heat treatment may be defined as an operation involving the heating of solid metal to definite temperature, followed by cooling at suitable rates in order to obtain certain physical properties which are associated with changes in the nature, form size and distribution of the micro-constituents.
1. Annealing:
Annealing is a het treatment process that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable.
Annealing consists of:
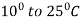
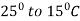
2. Normalizing:
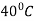
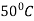
Question 2: Define Tempering. Explain various stages involved in Tempering.
A2: The tempering process provides a method for transforming martensite into ferrite and cementite. How much of the martensite is transformed depends on the temperature and time of the tempering process.
Tempering occurs in four stages:
Stage 1:
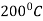
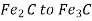
Stage 2:
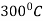
Stage 3:
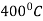
Stage 4:
Question 3: Discuss about the Speroidising.
A3: Spheroidising:
This is a form of annealing in which cementite is in the granular (globular) form is produced in the structure of steel.
This process causes the agglomeration of all carbides in the steel in the form of small globules or spheroids.
This process is usually applied to high-carbon steels which are difficult to machine.
The process consists of heating the steel slightly above the lower critical point (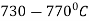 ) holding at this temperature, and then cooling slowly to a temperature of
) holding at this temperature, and then cooling slowly to a temperature of 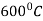 .
.
The rate of cooling in the furnace is from 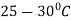 per hour.
per hour.
Question 4: Explain Isothermal transformation diagram for Fe-C alloys and microstructure development:
A4: Isothermal transformation diagrams (also known as time-temperature-transformation (TTT) diagrams) are plots of temperature versus time (usually on a logarithmic scale).
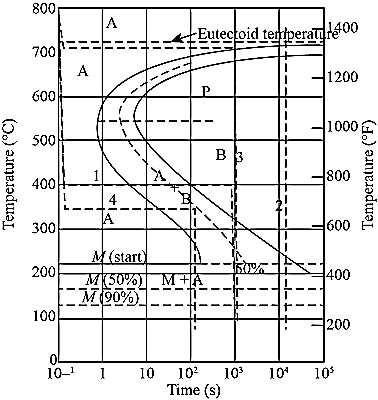
Figure: Isothermal Transformation Diagram
Question 5: Describe Continuous cooling curves:
A5: In actual practice steel is generally cooled continuously. Continuous cooling transformation (C-C-T) diagrams depict this situation.
The C-C-T curve (dark line) is shifted to the right of the T-T-T(dashed) curve as continuous cooling transformation occurs at lower temperature and longer time compared to isothermal bonding.
Bainite generally doesn’t form in steels during continuous cooling and hence the C-C-C-T curve ceases just below the nose.
The microstructure (fine or coarse) depends on the cooling rate. Higher the cooling rate finer the microstructure is.
The critical cooling rate is the one at which the cooling curve just touches the nose pf the C-C-T curve.
A cooling rate is higher than the critical rate is needed to form martensite.
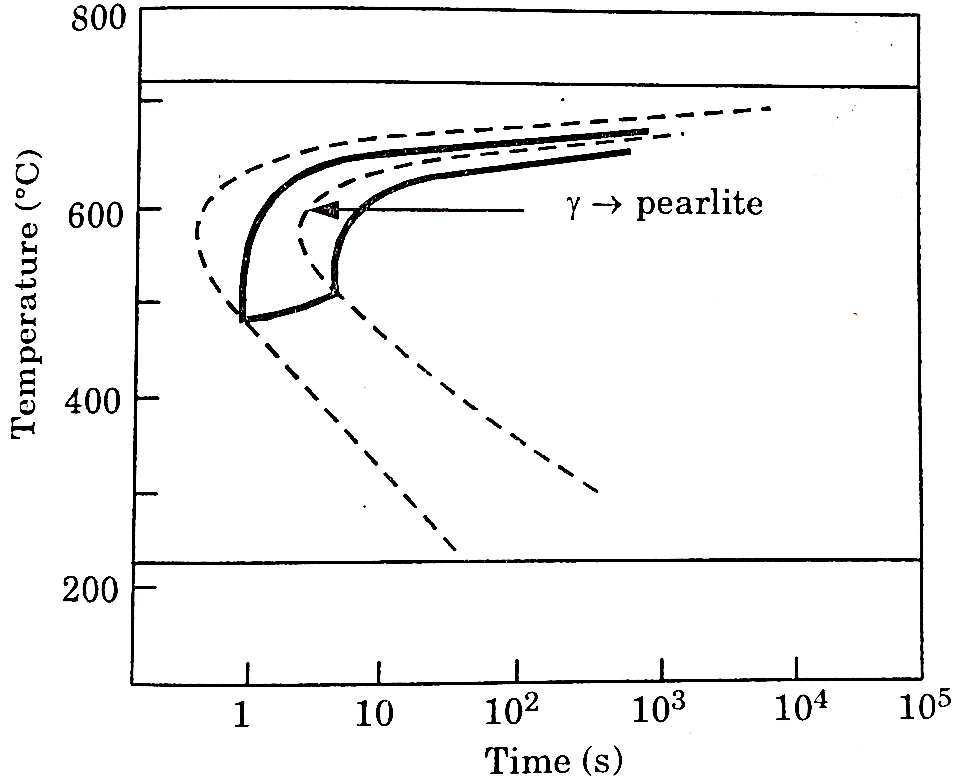
Figure: C-C-T Curve
Question 6: Describe the T-T-T diagram.
A6:
Temperature-Time -Transformation diagram represents a relation between starting and ending of the formation of different microstructures.
Its shape is name as English alphabet ‘C’ so it is known as C-curve.
The nose of this curve indicates the least time taken for a particular transformation.
The line passes through the nose (or tangent at the nose of C-curve) is known as critical cooling curves and its slope is termed as critical cooling rate.
In this fig. transformation of austenite to pearlite is shown. The left most T- curve shows the starting of transformation of austenite into pearlite.
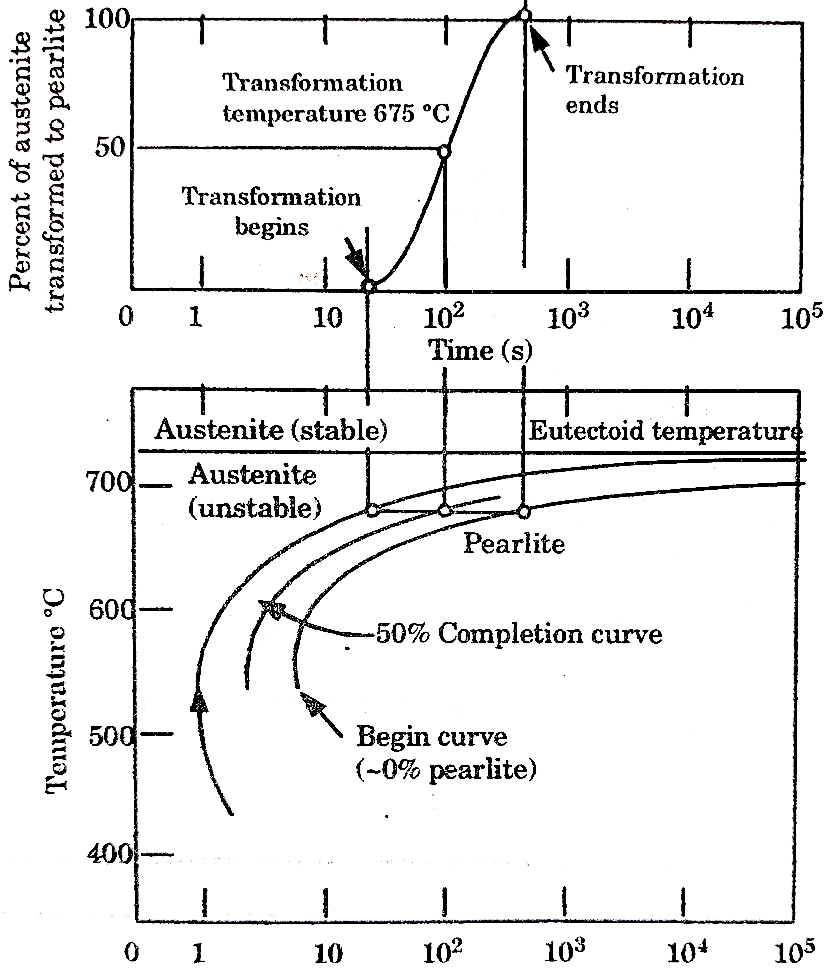
Figure: TTT diagram
At the 0% pearlite, 100%austenite is present. Then the dashed curve represents that 50% of austenite transforms into pearlite and the right most C-curve represents (completion curve) 100% pearlite transformation.
Question 7: Explain the importance of T-T-T Diagram.
A7: Importance of T-T-T Diagram:
It shows the structure that obtained at different cooling rates.
It graphically describes the cooling rate required for the transformation of austenite to pearlite, bainite or martensite.
It shows the time required for transformation to various phases.
Question 8: Explain the Austempring.
A8: Austempering:
It is very similar to martempering. Steel is austenitized and then quenched in a salt bath maintained at a constant temperature in the range of 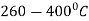 .
.
The article is held at this temperature for long enough to allow isothermal transformation to be completed.
After the complete transformation of austenite to bainite, steel is cooled to room temperature in air. It is also called isothermal quenching.
The temperature of quenching lies below the nose of the TTT curve and above the 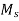 temperature.
temperature.
Heat treatment cycle for austempering is shown in figure 5.
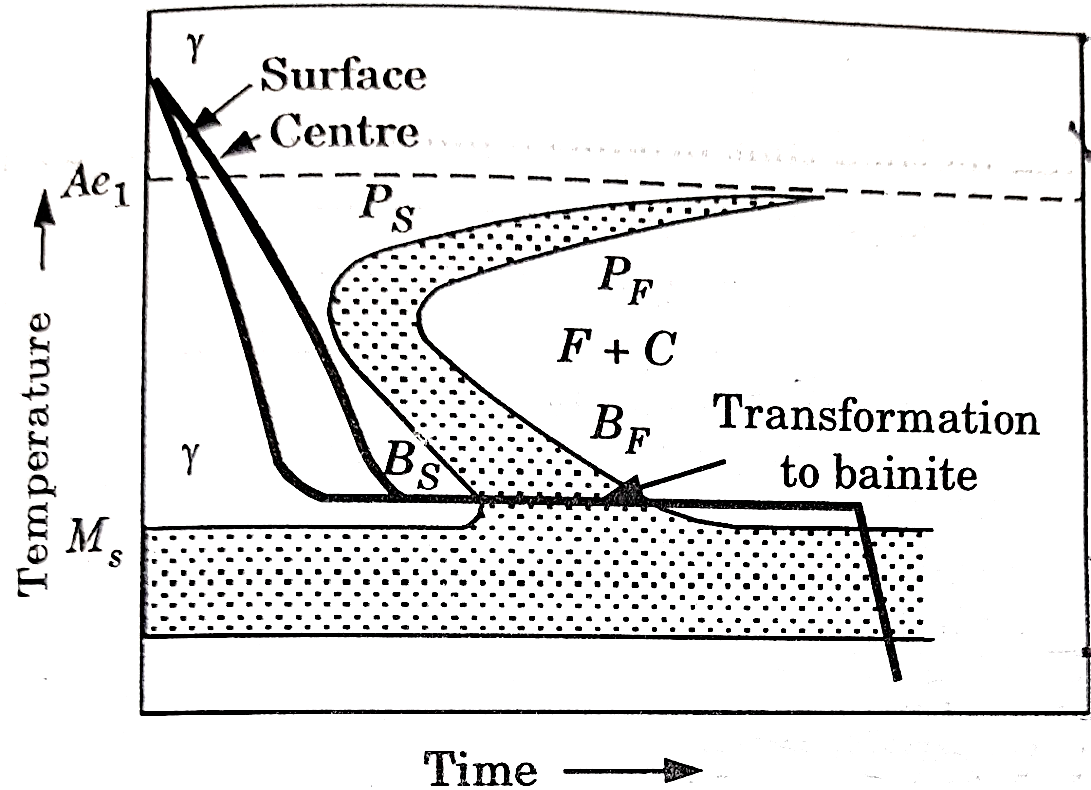
Figure: Heat Treatment cycle for Austempering
The principal purpose of austempering is to obtain high impact strength and increased notch toughness at a given high hardness level.
Question 9: Define the term Martempering.
A9: Martempering:
This is a hardening method that produces martensite. This method is also known as hardening by interrupted quenching
First the steel is heated to the hardening temperature then quenched in a medium (salt bath) having a temperature slightly above the point where martensite starts to form (usually from 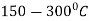 .
.
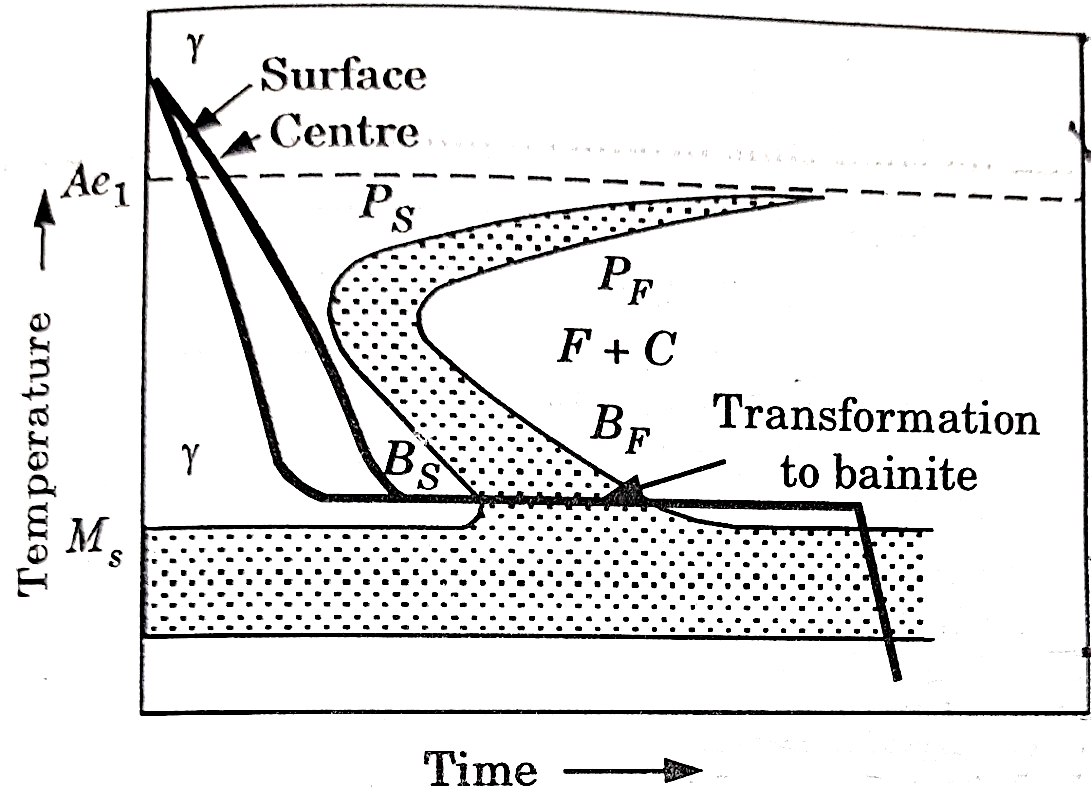
Figure: Heat Treatment cycle for martempering
It is held until it reaches the temperature of the medium and then cooled further to room temperature in air or oil. The holding time in quenching medium or bath should be sufficient to enable a uniform temperature to be reduced throughout the cross section but not long enough to cause austenite decomposition.
Austenite is transformed into martensite during the subsequent period of cooling to room temperature.
This treatment provides a structure of martensite and retained austenite in the hardening steel.
Question 10: Define the term Induction and Flame hardening.
A10: Flame and Induction Hardening:
Flame Hardening-
This process is based on rapid heating and quenching in order to produce a hard surface and soft core in the work.
An oxy-acetylene flame is used to heat the work above its critical temperature and quenching is done by means of a spray of water directed on the surface.
The torch for heating the work may be stationary or may move progressively over the work which may or may not spin as shown in figure given beow.
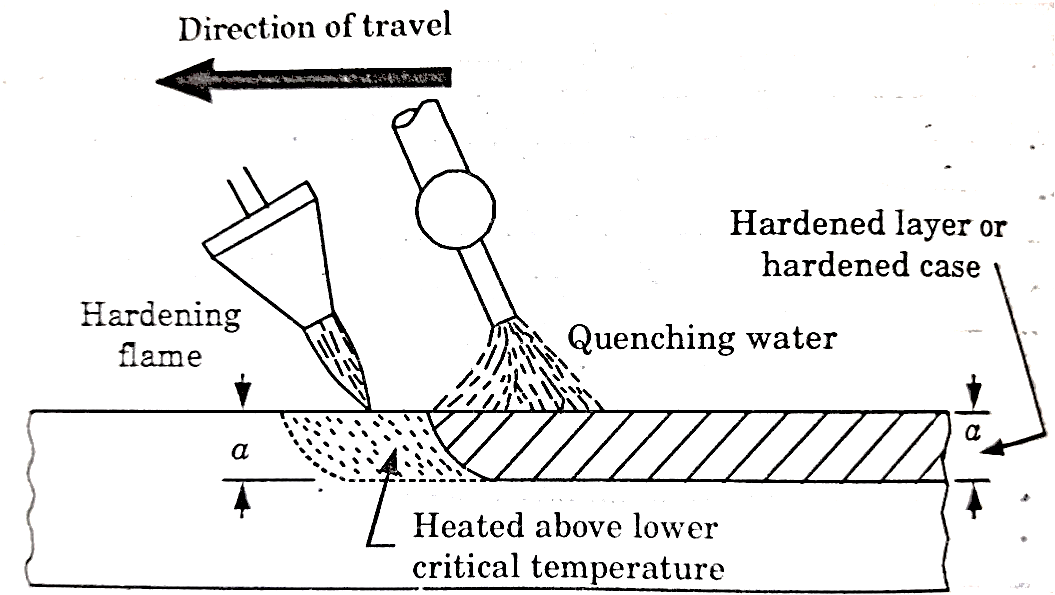
Figure: Flame Hardening
This method is applied for hardening cast gears, mill rolls or worms.
Induction Hardening-
It is a process of surface hardening in which the surface to be hardened is surrounded by copper inductor through which a high frequency current of about 2000 cycles per second is passed.
The inductor acts a primary coil of a transformer.
The work to be hardened is placed in the inductor in such a way that it does not touch the inductor as shown in fig.
The inductor block has a number of holes to spray water for quenching. The heating effect in the work is produced by the induced eddy currents and hysteresis loss in the surface of the work.
Steels containing 0.35-0.55% carbon are most frequently induction hardened. The hardening temperature is above  (Curie point) in order to increase the depth of current penetration.
(Curie point) in order to increase the depth of current penetration.
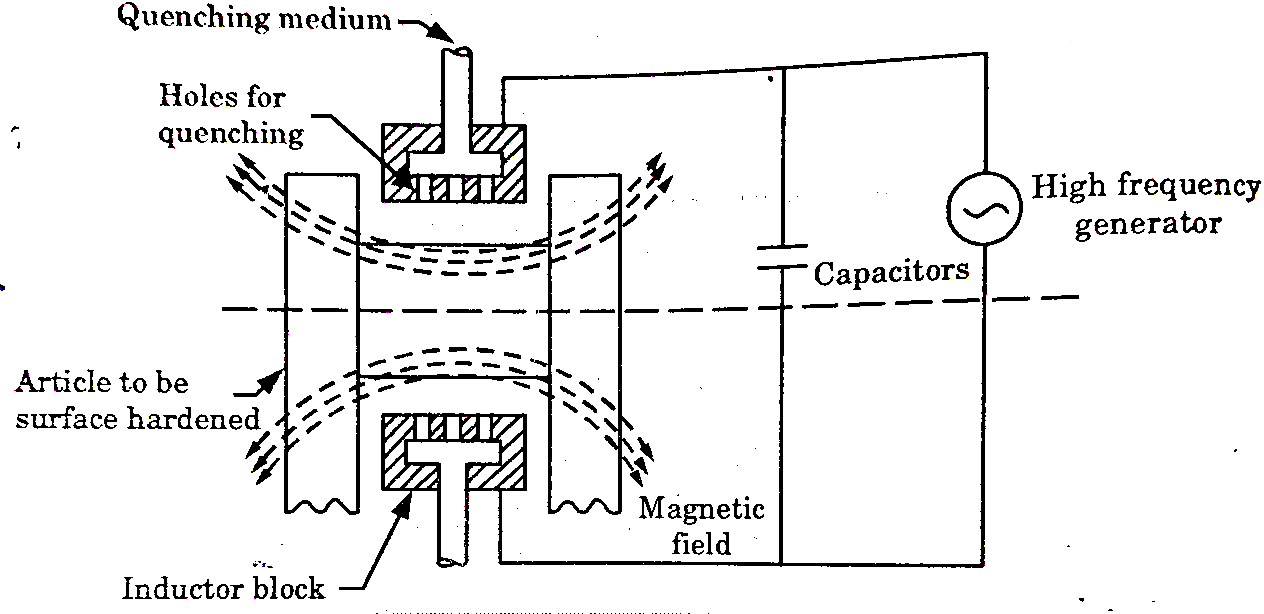
Figure: Arrangement for high- frequency induction heating
The heated areas are quenched immediately by water sprays directed from the inductor.
Induction hardening is, extensively used in many industrial plants.