Unit 3
Fuels
Q1) Write a short note on classification of fuels?
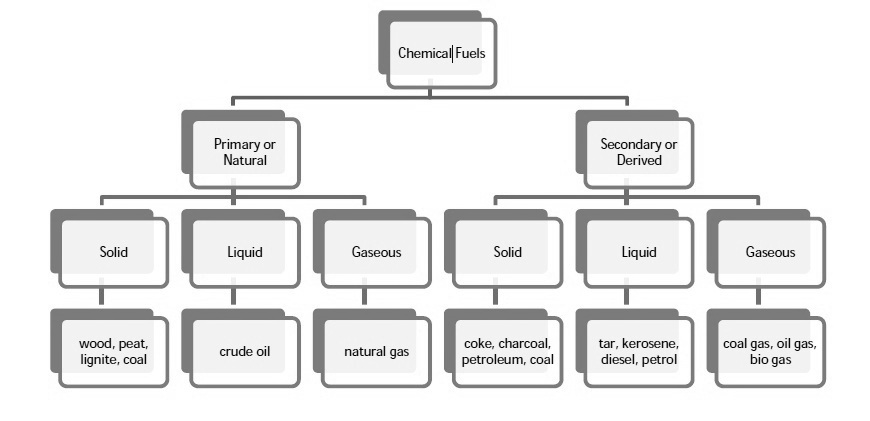
A1) Chemical fuels are classified on the basis of occurrence into :-
The secondary fuels are obtained from primary fuel by processing or they are manmade. e.g.:- charcoal is obtained from wood by partial combustion of wood , ethyl alcohol is obtained by fermentation of carbohydrates .
Both the primary and secondary fuels are further classified on the basis of physical state into solid liquid and gaseous fuels.
Extra definitions :-
1] fuels :-
Fuels are defined as materials that create usable energy through chemical nuclear or electrochemical reaction.
2] chemical fuel :-
The fossil fuels , wood vegetal oils etc. . which produce heat on burning are known as chemical fuels.
Q2) What do you mean by Calorific value?
A2) Calorific value :- efficiency of combustible fuels can be measured in terms of their calorific value . it is the heat evolved on combustion of a unit quantity of fuel.
Definition of calorific value :-
Q3) Write a brief deception about G.C.V. and N.C.V?
A3) Usually fuel contains some hydrogen . the hydrogen atoms are bonded to carbon atoms in the fuel when the fuel is burnt , hydrogen forms water vapors . the water vapors if cooled , we get certain amount of heat as the water has high latent heat of 2450 joules / gm or 587 Cal / gm.
Thus, during the study of calorific value of a fuel we get some heat directly by combustion of fuel and in addition we get certain amount of heat by cooling the products of combustion to 15-degree c.
Gross calorific value :-
Net calorific value :-
Net calorific value :-
The N.C.V and G.C.V are related as under
G.C.V = N.C.V + 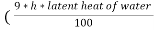 )
)
Where h is the percentage of hydrogen in the fuel.
It should be noted that the unit of latent heat of water and unit of G.V.C , N.C.V should be same .
Units of calorific value :-
System | Solid or liquid fuel | Gaseous fuel |
CGS | Cal / gm | Cal / liter |
MKS | Kcal / kg | Kcal / |
S.I | Joules / kg | Joules / |
Q4) Explain what do you mean by solid fuels?
A4) Solid fuels :- coal
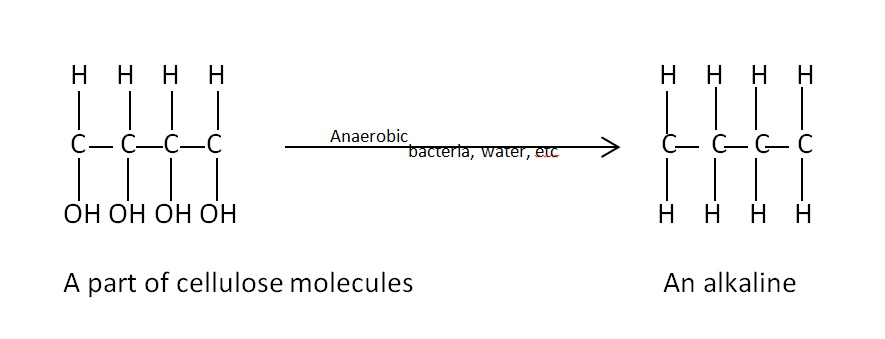
Coal is highly carbeanous matter formed vegetable matter buried in geomorphic changes , under pressure , by action of aerobic and anaerobic bacteria for long time.
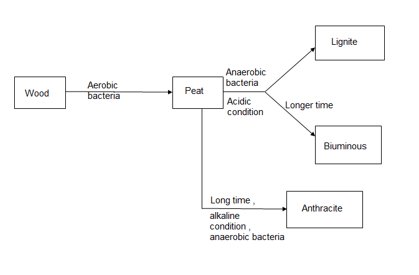
Peat :-
Lignite :-
Bituminous coal :-
Anthracite coal :-
Q5) Write a short note on the analysis of coal?
A5)
Given data :-
Increase in weight of any hydrous calcl2 = 0.55
Mass of coal = 0.75gms
Volume of 0.12 NHCL consumed by NH3 = 50 – 41
= 9 ml
Mass of coal = 1.8gms
Weight of Baso4 = 0.31 gm
Therefore ,
C percent ) = 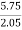 *
*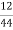 *100 = 76.5 percent .
*100 = 76.5 percent .
Increase in weight of any hydrous Cacl2
= weight of H2O formed
Mass of coal = 2.05gm
H ( percent ) = 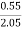 *
*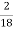 *100
*100
= 2.98 ( percent )
2. N ( percent ) = 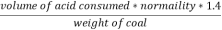
= 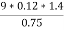
= 2.02 ( percent )
3. S ( percent ) = 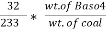 *100
*100
= 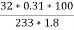
= 2.36 ( percent )
Theoretical calorific values
A] gouthal formula :-
Theoretically calorific value of coal can be calculated from proximate analysis data by using gouthal formula .
G.C.V Cal / gm = 82fc + a.VM
The values of constant are as under
Q6) What is the classification of petroleum?
A6) Petroleum :-
Origin of petroleum :-
Composition of petroleum :-
Average elemental composition of petroleum crude oil is,
Petroleum contains following type of compounds :-
1) Open chain alkanes
2) Cycloalkanes
3) Aromatics
4) Asphaltenes
5) Resins
Open chain alkenes :-
Both straight chain and branched alkanes are present in crude oil. In some crude oil such alkenes are major part.
Aromatics :-
Asphaltenes :-
Resins :-
Refining of petroleum :-
Q7) What is the process for Refining of petroleum?
A7) The various petroleum fraction has calorific value about 1100 Cal / gm .their composition, boiling range and uses are given.
Name of fraction | Boiling range | Composition of HC | uses |
| Below 40 ° c | C1 to c4 | Domestic and industrial fuel under lpg name |
2. Petroleum ether | 40-70° c | C5 to c7 | Fuel for Aeroplan helicopter as solvent |
3. Petrol or gasoline | 60-120° c | C5 to c8 | Fuel for petrol engines dry cleaning as solvent. |
4. Naphtha or solvent spirit | 120 -180 ° c | C7 to C10 | For dry cleaning for chemicals |
5. Kerosene | 180-250° c | C10 TO C16 | For domestic fuel for oil gas. |
6. Diesels | 250 - 320° c | C15 to c18 | Diesel engine fuel |
7. Heavy oil
| 320-400° c | C18 – c20
| Lubricating purpose |
8. residue |
| Above C30 | Road making water proofing |
Q8) Short note power alcohol.
A8) Power alcohol
Definition :-
When ethyl alcohol is used as fuel internal combustion engine .it is called as power alcohol . combustion engine it is called as power alcohol generally , ethyl alcohol is used as its 5 – 25 percent mixture with petrol.
Reactions :-
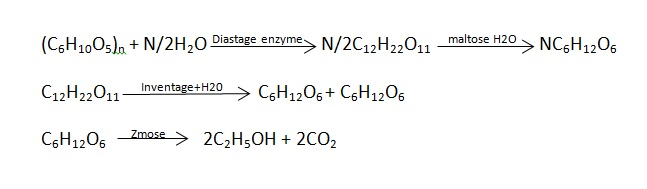
4. The starch or sucrose in molasses is converted into ethyl alcohol by fermentation as in above reactions the ethyl alcohol obtained contains 95.5 percent alcohol is separated by use of suitable dehydration agent or by distilling it along with benzene .
Advantages of power alcohol :-
Therefore, addition of ethyl alcohol to petrol increases its octane number.
2. Alcohol has property of absorbing any traces of water if present in petrol.
3. If a specially designed engine with higher compression ratio is used, then the disadvantage of lower c.v of ethyl alcohol can be overcome.
4. Ethyl alcohol contains O atoms which help for complete combustion of power alcohol and the polluting emissions of co , hydrocarbon is reduced largely.
5. Use of ethyl alcohol in petrol reduces our dependence on foreign countries for petrol and saves foreign currency considerably.
6. Power alcohol is cheaper than petrol.
Disadvantages :-
2. Ethyl alcohol has high surface tension and its atomization especially at lower temperature is difficult causing starting trouble.
3. Ethyl alcohol may undergo oxidation to form acetic acid which corrodes engine parts .
4. Ethyl alcohol obtained by fermentation processes directlycannot be mixed with petrol, but it has to be dehydrated first.
5. Prepare sodium methoxide from sodium metal and methanol .add the sodium methoxide about 2 percent by weight to vegetable oil or fat.
6. Add methanol about 20 percent by volume to the mixture .
7. Heat the mixture with stirring for 30 min
8. Cool and mix sufficient water stir well the glycerol and soap dissolve in water phase .
9. Separate the water insoluble phase from water phase
10. Add antioxidant to the biodiesel to avoid it to become gumany due to oxidation and polymerization.
Q9) What do you mean by Biodiesel?
A9) Biodiesel can be obtained from various vegetable oils like soya beenoil ,palm oil , groundnut oil , cottonseed oil , mustard oil , flower oil , etc. . and also, from the animal fats .
The product is given name biodiesel on account of that it is obtained from biological product and It is biodegradable material.
Advantages of biodiesel:-
Biodiesel can be used as a good fuel for diesel engine but generally it is used as its 20 percent mixture with diesel.
2. It has high c.v of about 40 kg / gm
3. It is regenerative and environmentally friendly .
4. It does not give out particulate and CO pollutants .
5. It has certain extent of lubricity
6. It uses provided good market to vegetable oils and reduces our dependence on biodiesel onforeign countries , saving currency.
7. It is clean to use biodiesel in diesel engine.
Q10) What do you mean by gaseous fuel? Explain it briefly?
A10) Natural Gas is a fossil fuel. It occurs naturally and approximately consists of 95% hydrocarbon methane, the other 5% consists of nitrogen, carbon dioxide, helium or hydrogen sulfide. It takes millions of years for it to form.The gas is formed when layers of decaying plants and animals are buried under the earth’s surface and are exposed to intense heat and pressure for millions of years. Plants originally obtain energy from the sun and store in the form of chemical bonds in the gas. And thus, the formation of this gas occurs.
Properties:
Applications
Hydrogen gas as a future fuel
The main purposes to produce green hydrogen is to add it to the natural gas system, because the natural gas system is seen as a seasonal and large energy storage and necessary for balancing the intermittent electricity production from renewable wind and solar. When hydrogen is mixed with natural gas, the hydrogen content can vary in a wide range. Gas engines have the capability to burn gases with a wide range of hydrogen content, but there are also some challenges with a potentially high fluctuation of the hydrogen content or fast rate of change.
The two technology areas, traffic decarbonisation and long-term energy storage, depend on H2. As H2 will be transported in pipelines, the need for flexible H2-tolerant gas engines will rise.