Unit 04
Q-What is atomic radii in periodic table?
Atomic radius is the radius of spherical atoms. Nonbonding atoms have a larger, more undefined radius, so when atomic radius is discussed as a periodic trend, what's usually meant is bonding atomic radius. These are the radius of atoms that are chemically bonded to one another. So, if the bond between two Cl atoms in Cl2 is 1.99 angstroms, we report chlorine's bonding atomic radius as about 0.99 angstroms. Further these values to estimate bond lengths between different elements in molecules.
Q-What is ionic radii in periodic table?
Ionic radii are the radii of ions of elements. These distances are based on distances between ions in ionic compounds. Cations, or positively charged ions, are smaller than their "parent" atoms. This is because cations are formed when those outermost orbitals are vacated of electrons. This also decreases electron-electron repulsions. Therefore, the resulting ions are smaller as there are not as many occupied orbitals and the effective nuclear charge affecting the remaining electrons increases, pulling electrons in more closely.
Q-Explain Ionization energy and its trends in periodic table.
Ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation.
H(g)→H+(g)+e−(1)(1)H(g)→H+(g)+e−
This energy is usually expressed in kJ/mol.
When considering an initially neutral atom, expelling the first electron will require less energy than expelling the second, the second will require less energy than the third, and so on. Each successive electron requires more energy to be released. This is because after the first electron is lost, the overall charge of the atom becomes positive, and the negative forces of the electron will be attracted to the positive charge of the newly formed ion. The more electrons that are lost, the more positive this ion will be, the harder it is to separate the electrons from the atom.
Periodic Table and Trend of Ionization Energies
Ionization energies are dependent upon the atomic radius. Since going from right to left on the periodic table, the atomic radius increases, and the ionization energy increases from left to right in the periods and up the groups. Exceptions to this trend are observed for alkaline earth metals (group 2) and nitrogen group elements (group 15). Typically, group 2 elements have ionization energy greater than group 13 elements and group 15 elements have greater ionization energy than group 16 elements. Groups 2 and 15 have completely and half-filled electronic configuration respectively, thus, it requires more energy to remove an electron from completely filled orbitals than incompletely filled orbitals.
Alkali metals (IA group) have small ionization energies, especially when compared to halogens group. In addition to the radius, the number of electrons between the nucleus and the electron in the outermost shell has an effect on the ionization energy as well. This effect, where the full positive charge of the nucleus is not felt by outer electrons due to the negative charges of inner electrons partially canceling out the positive charge, is called shielding. The more electrons shielding the outer electron shell from the nucleus, the less energy required to expel an electron from said atom. The higher the shielding effect the lower the ionization energy. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. From this trend, Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy.
Q-Explain electron affinity.
Electron affinity is defined as the amount of energy released on the addition of electron in neutral atom to form an anion. The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favorable the electron addition process is. Not all elements forms stable negative ions in which case the electron affinity is zero or even positive.
Electron affinity increases going left to right across a period. The overall trend across a period occurs because of increased nuclear attraction.
Going down the group the electron affinity should decrease since the electron is being added increasingly further away from the atom. Less tightly bound and therefore closer in energy to a free electron.
Electron affinity=1/Atomic Size
1. Atomic size: If the atomic size is small, then there will be greater electron gain enthalpy because the effective nuclear forces will be greater in the smaller atoms and the electrons will be held firmly.
2. Nuclear charge: The greater the nuclear charge more will be the value for electron gain enthalpy because an increase in nuclear charge will increase the effective nuclear force on valence electrons.
Q-Explain electro negativity and its trends in periodic table.
It basically indicates the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs. Electro negativity can be measured on several scales. The most commonly used scale was designed by Linus Pauling. According to this scale, fluorine is the most electronegative element with a value of 4.0 and cesium is the least electronegative element with a value of 0.7.
Periodic Trends of electro negativity: On moving across a period from left to right the nuclear charge increases and the atomic size decreases, therefore the value of electro negativity increases across a period in the modern periodic table.
There is an increase in the atomic number as we move down the group in the modern periodic table. The nuclear charge also increases but the effect of the increase in nuclear charge is overcome by the addition of one shell. Hence, the value of electro negativity decreases as we move down the group. It is a general observation that metals show a lower value of electro negativity as compared to the non-metals.
Q-Explain the variations of s, p, d, f orbital.
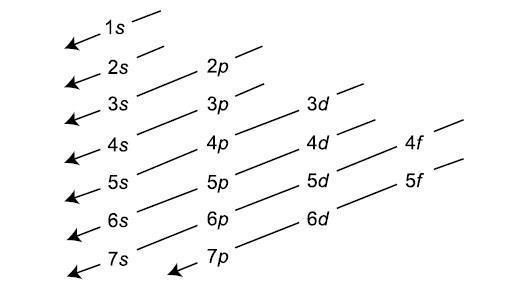
The Energy of the s, p , d and f orbital are related as, s < p < d < f (It is only true for a given value of the principal Quantum number). This Means Energy Increases in each and every orbital. s orbital have the lowest energy and the highest Penetration effect. But as we know, Energy continuously increases in each Block as we move downwards in group or left to right across the period. As the Energy of orbital has been determined by the n + l rule which is the basis of the Aufbau Principle. Aufbau Principle is the basic Principle based on the n + l rule which is used for filling the electrons from lower energy level to the higher Energy levels.
The Aufbau is the Greek word which means climb up. This Rule tells us that electrons climbs up from lower energy level to higher energy level.
This Principle has much significance, as it has made the method of arranging the electrons easy. The Electronic configuration which may be defined as the arrangement of the electrons in an atom is systematically told by the Aufbau Principle. The increasing orders of atomic orbital in which electrons will be filed are,
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p.
The Chart for determining the lower energy level and higher energy level and the way in which electrons will be filled is shown in attachment.
Q-What do you understand by Polarazibility?
Polarizability is a measurement of a tendency of electron cloud is distortion by an electric field. Typically the electron cloud will belong to an atom or molecule or ion. The electric field could be caused, E.g. By an electrode or a nearby cation or anion.
If an electron cloud is easy to distort, we say that the species it belongs to is polarizable.
Polarizability, which is represented by the Greek letter alpha, α, is experimentally measured as the ratio of induced dipole moment p to the electric field E that induces it:
α = p/E
The units of α are C m2 V-1.
Q-List the geometrical structure of different coordination number.
Coordination Number | Geometric Structure |
2 | Linear |
3 | T-shaped, Trigonal Pyramidal |
4 | Tetrahedral |
5 | Trigonal bipyramidal |
6 | Hexagonal Planar |
7 | Pentagonal bipyramidal |
8 | Hexagonal bipyramidal |
9 | Three face centered trigonal prism |
10 | Bi-capped square ant prism structure |
Q-Discuss about HSAB.
Pearson suggested a simple rule for predicting the stability of complexes formed between hard and soft acids and bases. “Hard acids prefer to bind (co-ordinate) with hard bases and soft acids prefer to bind with soft bases and gives stable complex compound”. It should be noted that the statement given above is not a theory or an explanation but it is simple rule of thumb which enables us to predict the relative stabilities of acid-bases adducts qualitatively.
Hard Acids and Bases
Hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom.
Examples of Hard Acids: H+, Li+, K+, Ca2+, Al3+, Sn4+, BF3, BCl3, CO2, RCO+, SO3, RMgX, VO2+, AlCl3
Hard bases are highly electronegative and of low polarizability.
Examples of Hard Bases: F-, OH-, NH3, N2H4, ROH, H2O, SO42-, PO43-
Hard bases react more readily to form stable compounds and complexes with hard acids.
Soft Acids and Bases
Soft acids consist of large low charge cations and molecules with relatively high energy occupied molecular orbitals. Soft acids are readily polarizable.
Examples of Soft Acids: Cs+, Cu+, Au+, Pt2+, Hg+, BH3, Br2, I2, RO+, quinones
Hard bases have low electronegative and low polarizability.
Examples of Soft Bases: H-, R-, CO, PR3, C6H6, SCN-
Soft bases react more readily and form stable compounds and complexes with soft acids.
Q- Discuss about the characteristics of HSAB.
Hard acid soft acid:
Hard Acid | Soft Acid |
Small iconic radius | Large iconic radius |
High positive charge | Low positive charge |
Low electro negativity | Intermediate electro negativity |
High energy LUMO | Low energy LUMO |
Hard base soft base:
Hard Base | Soft Base |
Small radius | Large radius |
High electro negativity | Intermediate electro negativity |
Weak Polarazibility | High Polarazibility |
High energy HOMO | Low energy HOMO |
Q-Using shielding and effective nuclear charge, explain the following.
a. The atomic radius of Cl is smaller than that of P.
b. Fe and Ni have similar atomic radii?
For both parts, look at the electron configuration and do a rough estimate of the effective nuclear charge:
Zeff = Z – σ.
Where σ is “shielding” from the inner shell electrons.
- Cl: [Ne]3s2 3p5 Zeff = 17-10=7 (There are 10 electrons in the inner, neon, core.)
P: [Ne]3s2 3p3 Zeff = 15-10=5 The valence shell electrons in chlorine feel a pull of a +7 from the nucleus. The valence shell electrons in phosphorus feel a pull of a +5 from the nucleus. The stronger the attractive force from the larger effective nuclear charge, the smaller the atom. Across a period (in the representative elements), the nuclear charge increases as the shielding remains constant. Therefore Cl is smaller than P – the trend is that the atoms will decrease in size from left to right across the periodic table for this reason.
b. Fe: [Ar]4s2 3d6 The electrons in the 4s subshell are the farthest away from the nucleus. Shielding will occur from the electrons in the Ar inner core as well as the six electrons in the 3d subshell. For Fe, Zeff = 26 – (18+6) = 2 Ni: [Ar]4s2 3d8 Zeff = 28 – (18+8) = 2 The effective nuclear charge is the roughly the same for both atoms. Therefore the size trend flattens out across the transition metals.
Unit 04
Q-What is atomic radii in periodic table?
Atomic radius is the radius of spherical atoms. Nonbonding atoms have a larger, more undefined radius, so when atomic radius is discussed as a periodic trend, what's usually meant is bonding atomic radius. These are the radius of atoms that are chemically bonded to one another. So, if the bond between two Cl atoms in Cl2 is 1.99 angstroms, we report chlorine's bonding atomic radius as about 0.99 angstroms. Further these values to estimate bond lengths between different elements in molecules.
Q-What is ionic radii in periodic table?
Ionic radii are the radii of ions of elements. These distances are based on distances between ions in ionic compounds. Cations, or positively charged ions, are smaller than their "parent" atoms. This is because cations are formed when those outermost orbitals are vacated of electrons. This also decreases electron-electron repulsions. Therefore, the resulting ions are smaller as there are not as many occupied orbitals and the effective nuclear charge affecting the remaining electrons increases, pulling electrons in more closely.
Q-Explain Ionization energy and its trends in periodic table.
Ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation.
H(g)→H+(g)+e−(1)(1)H(g)→H+(g)+e−
This energy is usually expressed in kJ/mol.
When considering an initially neutral atom, expelling the first electron will require less energy than expelling the second, the second will require less energy than the third, and so on. Each successive electron requires more energy to be released. This is because after the first electron is lost, the overall charge of the atom becomes positive, and the negative forces of the electron will be attracted to the positive charge of the newly formed ion. The more electrons that are lost, the more positive this ion will be, the harder it is to separate the electrons from the atom.
Periodic Table and Trend of Ionization Energies
Ionization energies are dependent upon the atomic radius. Since going from right to left on the periodic table, the atomic radius increases, and the ionization energy increases from left to right in the periods and up the groups. Exceptions to this trend are observed for alkaline earth metals (group 2) and nitrogen group elements (group 15). Typically, group 2 elements have ionization energy greater than group 13 elements and group 15 elements have greater ionization energy than group 16 elements. Groups 2 and 15 have completely and half-filled electronic configuration respectively, thus, it requires more energy to remove an electron from completely filled orbitals than incompletely filled orbitals.
Alkali metals (IA group) have small ionization energies, especially when compared to halogens group. In addition to the radius, the number of electrons between the nucleus and the electron in the outermost shell has an effect on the ionization energy as well. This effect, where the full positive charge of the nucleus is not felt by outer electrons due to the negative charges of inner electrons partially canceling out the positive charge, is called shielding. The more electrons shielding the outer electron shell from the nucleus, the less energy required to expel an electron from said atom. The higher the shielding effect the lower the ionization energy. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. From this trend, Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy.
Q-Explain electron affinity.
Electron affinity is defined as the amount of energy released on the addition of electron in neutral atom to form an anion. The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favorable the electron addition process is. Not all elements forms stable negative ions in which case the electron affinity is zero or even positive.
Electron affinity increases going left to right across a period. The overall trend across a period occurs because of increased nuclear attraction.
Going down the group the electron affinity should decrease since the electron is being added increasingly further away from the atom. Less tightly bound and therefore closer in energy to a free electron.
Electron affinity=1/Atomic Size
1. Atomic size: If the atomic size is small, then there will be greater electron gain enthalpy because the effective nuclear forces will be greater in the smaller atoms and the electrons will be held firmly.
2. Nuclear charge: The greater the nuclear charge more will be the value for electron gain enthalpy because an increase in nuclear charge will increase the effective nuclear force on valence electrons.
Q-Explain electro negativity and its trends in periodic table.
It basically indicates the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs. Electro negativity can be measured on several scales. The most commonly used scale was designed by Linus Pauling. According to this scale, fluorine is the most electronegative element with a value of 4.0 and cesium is the least electronegative element with a value of 0.7.
Periodic Trends of electro negativity: On moving across a period from left to right the nuclear charge increases and the atomic size decreases, therefore the value of electro negativity increases across a period in the modern periodic table.
There is an increase in the atomic number as we move down the group in the modern periodic table. The nuclear charge also increases but the effect of the increase in nuclear charge is overcome by the addition of one shell. Hence, the value of electro negativity decreases as we move down the group. It is a general observation that metals show a lower value of electro negativity as compared to the non-metals.
Q-Explain the variations of s, p, d, f orbital.
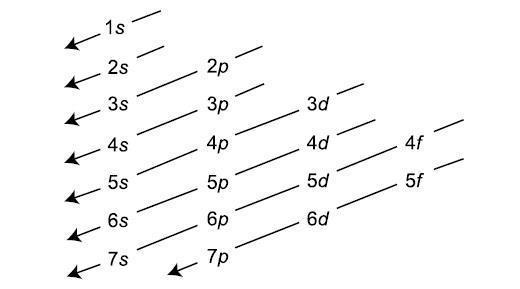
The Energy of the s, p , d and f orbital are related as, s < p < d < f (It is only true for a given value of the principal Quantum number). This Means Energy Increases in each and every orbital. s orbital have the lowest energy and the highest Penetration effect. But as we know, Energy continuously increases in each Block as we move downwards in group or left to right across the period. As the Energy of orbital has been determined by the n + l rule which is the basis of the Aufbau Principle. Aufbau Principle is the basic Principle based on the n + l rule which is used for filling the electrons from lower energy level to the higher Energy levels.
The Aufbau is the Greek word which means climb up. This Rule tells us that electrons climbs up from lower energy level to higher energy level.
This Principle has much significance, as it has made the method of arranging the electrons easy. The Electronic configuration which may be defined as the arrangement of the electrons in an atom is systematically told by the Aufbau Principle. The increasing orders of atomic orbital in which electrons will be filed are,
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p.
The Chart for determining the lower energy level and higher energy level and the way in which electrons will be filled is shown in attachment.
Q-What do you understand by Polarazibility?
Polarizability is a measurement of a tendency of electron cloud is distortion by an electric field. Typically the electron cloud will belong to an atom or molecule or ion. The electric field could be caused, E.g. By an electrode or a nearby cation or anion.
If an electron cloud is easy to distort, we say that the species it belongs to is polarizable.
Polarizability, which is represented by the Greek letter alpha, α, is experimentally measured as the ratio of induced dipole moment p to the electric field E that induces it:
α = p/E
The units of α are C m2 V-1.
Q-List the geometrical structure of different coordination number.
Coordination Number | Geometric Structure |
2 | Linear |
3 | T-shaped, Trigonal Pyramidal |
4 | Tetrahedral |
5 | Trigonal bipyramidal |
6 | Hexagonal Planar |
7 | Pentagonal bipyramidal |
8 | Hexagonal bipyramidal |
9 | Three face centered trigonal prism |
10 | Bi-capped square ant prism structure |
Q-Discuss about HSAB.
Pearson suggested a simple rule for predicting the stability of complexes formed between hard and soft acids and bases. “Hard acids prefer to bind (co-ordinate) with hard bases and soft acids prefer to bind with soft bases and gives stable complex compound”. It should be noted that the statement given above is not a theory or an explanation but it is simple rule of thumb which enables us to predict the relative stabilities of acid-bases adducts qualitatively.
Hard Acids and Bases
Hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom.
Examples of Hard Acids: H+, Li+, K+, Ca2+, Al3+, Sn4+, BF3, BCl3, CO2, RCO+, SO3, RMgX, VO2+, AlCl3
Hard bases are highly electronegative and of low polarizability.
Examples of Hard Bases: F-, OH-, NH3, N2H4, ROH, H2O, SO42-, PO43-
Hard bases react more readily to form stable compounds and complexes with hard acids.
Soft Acids and Bases
Soft acids consist of large low charge cations and molecules with relatively high energy occupied molecular orbitals. Soft acids are readily polarizable.
Examples of Soft Acids: Cs+, Cu+, Au+, Pt2+, Hg+, BH3, Br2, I2, RO+, quinones
Hard bases have low electronegative and low polarizability.
Examples of Soft Bases: H-, R-, CO, PR3, C6H6, SCN-
Soft bases react more readily and form stable compounds and complexes with soft acids.
Q- Discuss about the characteristics of HSAB.
Hard acid soft acid:
Hard Acid | Soft Acid |
Small iconic radius | Large iconic radius |
High positive charge | Low positive charge |
Low electro negativity | Intermediate electro negativity |
High energy LUMO | Low energy LUMO |
Hard base soft base:
Hard Base | Soft Base |
Small radius | Large radius |
High electro negativity | Intermediate electro negativity |
Weak Polarazibility | High Polarazibility |
High energy HOMO | Low energy HOMO |
Q-Using shielding and effective nuclear charge, explain the following.
a. The atomic radius of Cl is smaller than that of P.
b. Fe and Ni have similar atomic radii?
For both parts, look at the electron configuration and do a rough estimate of the effective nuclear charge:
Zeff = Z – σ.
Where σ is “shielding” from the inner shell electrons.
- Cl: [Ne]3s2 3p5 Zeff = 17-10=7 (There are 10 electrons in the inner, neon, core.)
P: [Ne]3s2 3p3 Zeff = 15-10=5 The valence shell electrons in chlorine feel a pull of a +7 from the nucleus. The valence shell electrons in phosphorus feel a pull of a +5 from the nucleus. The stronger the attractive force from the larger effective nuclear charge, the smaller the atom. Across a period (in the representative elements), the nuclear charge increases as the shielding remains constant. Therefore Cl is smaller than P – the trend is that the atoms will decrease in size from left to right across the periodic table for this reason.
b. Fe: [Ar]4s2 3d6 The electrons in the 4s subshell are the farthest away from the nucleus. Shielding will occur from the electrons in the Ar inner core as well as the six electrons in the 3d subshell. For Fe, Zeff = 26 – (18+6) = 2 Ni: [Ar]4s2 3d8 Zeff = 28 – (18+8) = 2 The effective nuclear charge is the roughly the same for both atoms. Therefore the size trend flattens out across the transition metals.