Question Bank
Unit 10
Question Bank
Unit 10
Q-What are the common features of reactive intermediates?
Reactive intermediates have several features in common:
- Low concentration with respect to reaction substrate and final reaction product
- Often generated on chemical decomposition of a chemical compound
- It is often possible to prove the existence of this species by spectroscopic means
- Cage effects have to be taken into account
- Often stabilization by conjugation or resonance
- Often difficult to distinguish from a transition state
- Prove existence by means of chemical trapping
Q-What is elimination reaction?
Elimination reaction is the type of reaction that is mainly used to convert saturated compounds to the unsaturated compounds that is organic compounds which contain single carbon bonds to the compound which features double or triple carbon bonds. It is a chemical reaction where several atoms either pair or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or action of metals. It can also happen through the process of heating at high temperatures.
Q-Discuss about the E1 reaction.
- This is also called as unimolecular elimination reaction, there are usually two steps involved – ionization and deprotonation.
- During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
- This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
- In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
- It exhibits first-order kinetics.
Q-What is rearrangement reaction?
The term “rearrangement” is used to describe two different types of organic chemical reactions. A rearrangement may involve the one step migration of an H atom or of a larger molecular fragment within a relatively short lived intermediate.
Q-What is curtius rearrangement reaction?
Curtius Rearrangement:
Curtius reaction involves the heating of an acyl azide which loses nitrogen and then rearranges to an isocyanate.
RCON3 → R-N=C=O + N2
If the reaction is performed in an alcoholic or aqueous medium, the isocyanate further reacts to form urethane, amine or substituted urea.
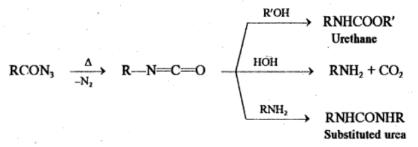

The conversion of acyl azides to isocyanates involves Curtius rearrangement whereas curtius reaction involves the conversion of acids to amines, urethane and substituted urea via Curtius rearrangement.
Acyl azide required for the reaction is obtained as follows.
RCOCl + NaN3 → RCON3 + NaCl
RCOOC2H5 → RCONHNH2 → RCON3 + 2H2O
Q-What is Claisen rearrangement reaction?
Claisen Rearrangement:
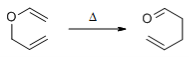
The aliphatic Claisen Rearrangement is a [3,3]-sigmatropic rearrangement in which an allyl vinyl ether is converted thermally to an unsaturated carbonyl compound.
The aromatic Claisen Rearrangement is accompanied by a rearomatization:
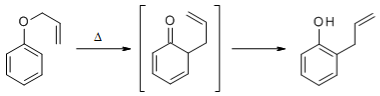
The etherification of alcohols or phenols and their subsequent Claisen Rearrangement under thermal conditions makes possible an extension of the carbon chain of the molecule.
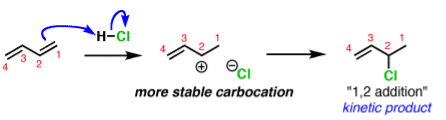
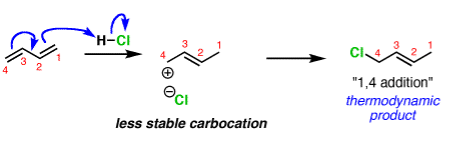
Q-Discuss about the kinetic and thermodynamic aspects of chemical equation with example.
In organic chemistry the two different products can form depending on the reaction conditions. This is called kinetic and thermodynamic control.
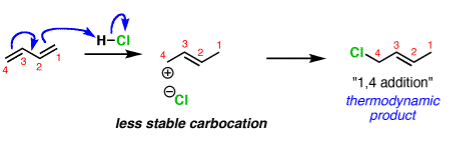
A common example is in additions of acids like HCl to dienes, such as butadiene.
The activation energy required to start the reaction. The activation energy is related to the stability of the carbocation that’s formed. Carbocations become more stabilized as the number of attached carbons increases. So the above example with a secondary carbocation is more stable than the bottom example primary carbocation is formed faster. This is the kinetic product.
The reliability of the can opener is similar to the thermodynamic stability of the alkene that is formed. Alkenes increase in stability with the number of attached carbons. So the “1,4” product (where H and Cl add to C-1 and C-4 in our example) is more stable than the “1,2” product (where H and Cl add to C-1 and C-2) since the alkene has two carbon substituents. This is the thermodynamic product.
And money is analogous to the available energy (i.e. temperature). At low temperatures, when not much energy is available, the kinetic product will dominate. At high temperatures the thermodynamic product will be the major product.
Q-What are different type of solvents?
Hydrocarbon solvents are classified into three sub-groups based on the type of “carbon skeleton” of their molecules, giving us the aliphatic, aromatic and paraffinic solvents families. Paint thinner is a common example of a hydrocarbon solvent.
Oxygenated solvents are produced through chemical reactions from olefins, giving us the following sub-groups: alcohols, ketones, esters, ethers, glycol ethers and glycol ether esters. The human body naturally produces ketones when it burns fat.
Halogenated solvents are solvents that contain a halogen such as chlorine, bromine or iodine. Many people recognize perchloroethylene as an example – a highly effective solvent used in dry cleaning.
Q-Explain Pfizer Sertaline synthesis.

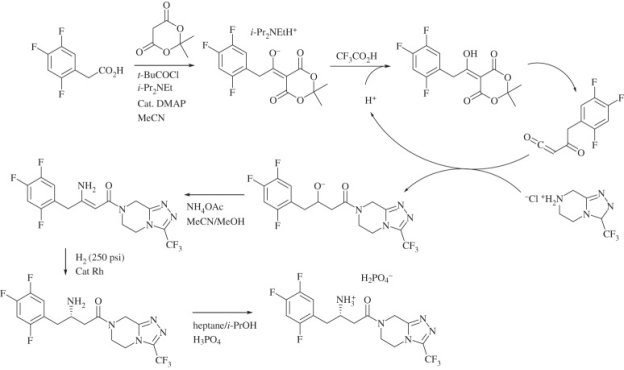
Q-Explain Mercks Sitagliptin synthesis.

Question Bank
Unit 10
Q-What are the common features of reactive intermediates?
Reactive intermediates have several features in common:
- Low concentration with respect to reaction substrate and final reaction product
- Often generated on chemical decomposition of a chemical compound
- It is often possible to prove the existence of this species by spectroscopic means
- Cage effects have to be taken into account
- Often stabilization by conjugation or resonance
- Often difficult to distinguish from a transition state
- Prove existence by means of chemical trapping
Q-What is elimination reaction?
Elimination reaction is the type of reaction that is mainly used to convert saturated compounds to the unsaturated compounds that is organic compounds which contain single carbon bonds to the compound which features double or triple carbon bonds. It is a chemical reaction where several atoms either pair or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or action of metals. It can also happen through the process of heating at high temperatures.
Q-Discuss about the E1 reaction.
- This is also called as unimolecular elimination reaction, there are usually two steps involved – ionization and deprotonation.
- During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
- This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
- In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
- It exhibits first-order kinetics.
Q-What is rearrangement reaction?
The term “rearrangement” is used to describe two different types of organic chemical reactions. A rearrangement may involve the one step migration of an H atom or of a larger molecular fragment within a relatively short lived intermediate.
Q-What is curtius rearrangement reaction?
Curtius Rearrangement:
Curtius reaction involves the heating of an acyl azide which loses nitrogen and then rearranges to an isocyanate.
RCON3 → R-N=C=O + N2
If the reaction is performed in an alcoholic or aqueous medium, the isocyanate further reacts to form urethane, amine or substituted urea.
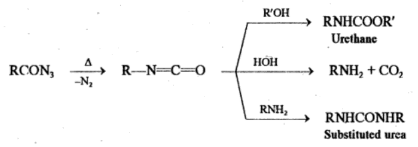

The conversion of acyl azides to isocyanates involves Curtius rearrangement whereas curtius reaction involves the conversion of acids to amines, urethane and substituted urea via Curtius rearrangement.
Acyl azide required for the reaction is obtained as follows.
RCOCl + NaN3 → RCON3 + NaCl
RCOOC2H5 → RCONHNH2 → RCON3 + 2H2O
Q-What is Claisen rearrangement reaction?
Claisen Rearrangement:

The aliphatic Claisen Rearrangement is a [3,3]-sigmatropic rearrangement in which an allyl vinyl ether is converted thermally to an unsaturated carbonyl compound.
The aromatic Claisen Rearrangement is accompanied by a rearomatization:
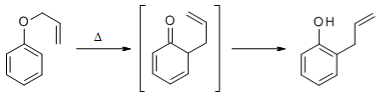
The etherification of alcohols or phenols and their subsequent Claisen Rearrangement under thermal conditions makes possible an extension of the carbon chain of the molecule.
Q-Discuss about the kinetic and thermodynamic aspects of chemical equation with example.
In organic chemistry the two different products can form depending on the reaction conditions. This is called kinetic and thermodynamic control.
A common example is in additions of acids like HCl to dienes, such as butadiene.
The activation energy required to start the reaction. The activation energy is related to the stability of the carbocation that’s formed. Carbocations become more stabilized as the number of attached carbons increases. So the above example with a secondary carbocation is more stable than the bottom example primary carbocation is formed faster. This is the kinetic product.
The reliability of the can opener is similar to the thermodynamic stability of the alkene that is formed. Alkenes increase in stability with the number of attached carbons. So the “1,4” product (where H and Cl add to C-1 and C-4 in our example) is more stable than the “1,2” product (where H and Cl add to C-1 and C-2) since the alkene has two carbon substituents. This is the thermodynamic product.
And money is analogous to the available energy (i.e. temperature). At low temperatures, when not much energy is available, the kinetic product will dominate. At high temperatures the thermodynamic product will be the major product.

Q-What are different type of solvents?
Hydrocarbon solvents are classified into three sub-groups based on the type of “carbon skeleton” of their molecules, giving us the aliphatic, aromatic and paraffinic solvents families. Paint thinner is a common example of a hydrocarbon solvent.
Oxygenated solvents are produced through chemical reactions from olefins, giving us the following sub-groups: alcohols, ketones, esters, ethers, glycol ethers and glycol ether esters. The human body naturally produces ketones when it burns fat.
Halogenated solvents are solvents that contain a halogen such as chlorine, bromine or iodine. Many people recognize perchloroethylene as an example – a highly effective solvent used in dry cleaning.
Q-Explain Pfizer Sertaline synthesis.

Q-Explain Mercks Sitagliptin synthesis.