Question Bank
Unit 07
Q-Enlist rules of LCAO.
Rules for the Linear Combination of Atomic Orbital are:-
- The combining atoms should have the same symmetry along the molecular axis for proper combination. e.g. All the sub-orbitals of 2p have same energy but still, the 2pz orbital of an atom can only combine with a 2pz orbital of another atom but cannot combine with 2px and 2py orbital as they have a different axis of symmetry.
- The two atomic orbital will combine to form molecular orbital. Greater is the extend of overlap of atomic orbital; greater will be the nuclear density.
- The combining atomic orbital must be of equal energy or approximately same energy.
Q--What is bonding molecular orbital.
When addition of wave function takes place, the type of molecular orbitals formed are called Bonding Molecular orbitals and is represented by
ΨMO = ΨA + ΨB.
They have lower energy than atomic orbitals involved. It is similar to constructive interference occurring in phase because of which electron probability density increases resulting in formation of bonding orbital. Molecular orbital formed by addition of overlapping of two s orbitals. It is represented by s.
Q- What is anti bonding molecular orbital?
When molecular orbital is formed by subtraction of wave function, the type of molecular orbitals formed are called Antibonding Molecular Orbitals and is represented by
ΨMO = ΨA - ΨB.
They have higher energy than atomic orbitals. It is similar to destructive interference occurring out of phase resulting in formation of antibonding orbitals. Molecular Orbital formed by subtraction of overlapping of two s orbitals. It is represented by s* (*) is used to represent antibonding molecular orbital) called Sigma Antibonding. Therefore, Combination of two atomic orbitals results in formation of two molecular orbitals, bonding molecular orbital (BMO) whereas other is anti-bonding molecular orbital (ABMO).
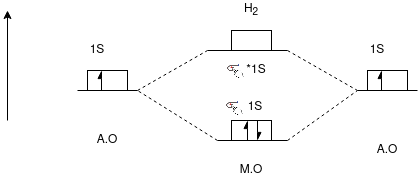
Q-Draw the energy level diagram of H2 molecule.
Q- Discuss about Carbon mono oxide with its energy level diagram, stabilization energy.
CO is the hetronuclear diatomic molecule. This molecule is formed by the combination of carbon and oxygen atom. Electronic configuration of carbon atom is 1s2, 2s2, 2p2 and that of oxygen is 1s2, 2s2, 2p4

Stabilisation Energy
[No. Of electron in BMO * (-) + No. Of electrons in anti bonding molecular orbital * (+)]
=(8) * (-) + (2) * (+)
= -6
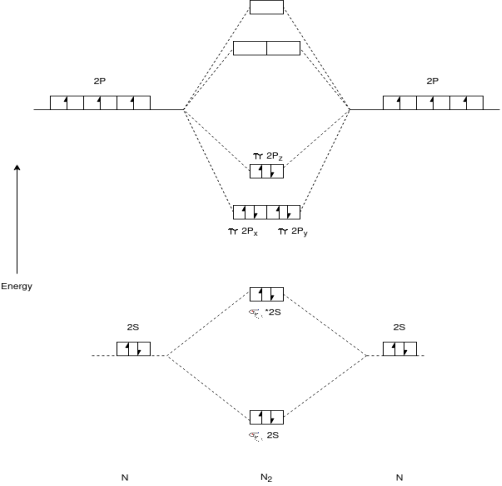
Q-Explain N2 molecule.
Q- Explain F2 molecule with its electronic configuration.

Q-Discuss about coordination chemistry.
The chemical structures in which a central metal atom is surrounded by nonmetal atoms or groups of atoms, called ligands, joined to it by chemical bonds. Coordination compounds include such substances as vitamin B12, hemoglobin, and chlorophyll, dyes and pigments, and catalysts used in preparing organic substances. A major application of coordination compounds is their use as catalysts, which serve to alter the rate of chemical reactions. Certain complex metal catalysts, E.g.:, they play a key role in the production of polyethylene and polypropylene. In addition, a very stable class of organ metallic coordination compounds has provided impetus to the development of organo metallic chemistry. Organ metallic coordination compounds are sometimes characterized by “sandwich” structures, in which two molecules of an unsaturated cyclic hydrocarbon, which lacks one or more hydrogen atoms, bond on either side of a metal atom. This results in a highly stable aromatic system.
Q-Write the nomenclature and IUPAC name of some compounds.
Common Name | IUPAC Name | Formula | Structure |
Acetylacetonato | 2,4-pentanediono | CH3COCHCOCH3 | 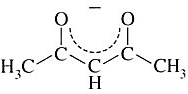 |
2,2-bipyridine | 2,2-bipyridine | Bipy | 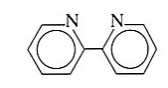 |
1,10-phenanthroline | 1,10-diaminophinanthrene | C12H8N2 | 
|
Dialkyldithiocarbamato | Dialklylcarbamodithioato | S2CNR2 |  |
Oxalate | Oxalate | C2O4 | 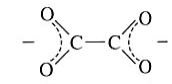 |
Dimethylglyoximato | Butanediene dioxime | HONCC(CH3)C(CH3)NO | 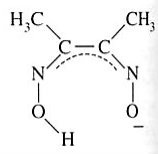 |
Q-Discuss about the spectra d-d transition.

 Many transition metal complexes show absorptions at relatively lower energies for electronic transitions (in the visible part of the spectrum). These are associated with transitions between d orbitals whose degeneracy has been removed by an environment of lower than spherical symmetry. E.g, in a octahedral complex, one may see transitions involving electron jumps from the t2g orbitals to the eg orbitals. These Orbitals are often only weakly bonding or antibonding - their energy separation is small. We may use the absorption energies to measure the ligand field splitting energies , but it should be noted that only in a few cases does the absorption energy correspond exactly to .
Many transition metal complexes show absorptions at relatively lower energies for electronic transitions (in the visible part of the spectrum). These are associated with transitions between d orbitals whose degeneracy has been removed by an environment of lower than spherical symmetry. E.g, in a octahedral complex, one may see transitions involving electron jumps from the t2g orbitals to the eg orbitals. These Orbitals are often only weakly bonding or antibonding - their energy separation is small. We may use the absorption energies to measure the ligand field splitting energies , but it should be noted that only in a few cases does the absorption energy correspond exactly to .
The study in which the transition metal complex ion absorbs electromagnetic radiation is called as the electron absorption spectroscopy. The absorbed radiation promotes electrons form the lower energy d-orbital to higher energy d-orbital. Such electronic transition are of high energy and in addition much lower energy vibrational and rotational transitions always occur. The vibrational and rotational levels are too close in energy. Therefore absorption bands due to high vibrational and rotational transitions are responsible for broadening of the electronic absorption in d-d spectra. Due to the promotion of electrons there occurs a change in the overall arrangement of electrons in d-orbital. The change in the overall arrangement of electrons in d-orbital gives rise to electron absorption spectrum.
The study of transition metal complex spectrum gives following:
(i) Color of complex
(ii) Magnitude of energy gap between ground state and excited state.
(iii) Extent of covalent character in metal ligand bond.
(iv) Geometry of the complex
The position of metal ions and ligand in spectrochemical series and nephelauxetic series.
Q-What is atomic spectroscopy?
Atomic spectroscopy deals with the consequences of inter electronic repulsion and magnetic interactions between electrons of an atom or an ion. These interactions involve the coupling of a spin and angular momentum of electrons within a given electronic configuration. The coupling rises several degenerate and non degenerate energy levels these energy levels are known as the energy terms or spectroscopic states. The electronic transition in these energy levels gives rise to electronic absorption spectrum.
Q- How to measure the electronic spectra of transition metal complexes?
The spectrum of a colored solution may be measured quite easily using a spectrophotometer. The beam of monochromatic light is passed through a solution and on to a photoelectric cell. The amount of light absorbed at any particular frequency can be read off or whole frequency range can be scanned. The absorbance If Io is the intensity of the original beam of light and I is the intensity after passing through the solution, then
A=log(Io/I)
The molar absorption coefficient is given by
E=A/Cl
Where,
C=concentration of solution
L= length
Question Bank
Unit 07
Question Bank
Unit 07
Question Bank
Unit 07
Q-Enlist rules of LCAO.
Rules for the Linear Combination of Atomic Orbital are:-
- The combining atoms should have the same symmetry along the molecular axis for proper combination. e.g. All the sub-orbitals of 2p have same energy but still, the 2pz orbital of an atom can only combine with a 2pz orbital of another atom but cannot combine with 2px and 2py orbital as they have a different axis of symmetry.
- The two atomic orbital will combine to form molecular orbital. Greater is the extend of overlap of atomic orbital; greater will be the nuclear density.
- The combining atomic orbital must be of equal energy or approximately same energy.
Q--What is bonding molecular orbital.
When addition of wave function takes place, the type of molecular orbitals formed are called Bonding Molecular orbitals and is represented by
ΨMO = ΨA + ΨB.
They have lower energy than atomic orbitals involved. It is similar to constructive interference occurring in phase because of which electron probability density increases resulting in formation of bonding orbital. Molecular orbital formed by addition of overlapping of two s orbitals. It is represented by s.
Q- What is anti bonding molecular orbital?
When molecular orbital is formed by subtraction of wave function, the type of molecular orbitals formed are called Antibonding Molecular Orbitals and is represented by
ΨMO = ΨA - ΨB.
They have higher energy than atomic orbitals. It is similar to destructive interference occurring out of phase resulting in formation of antibonding orbitals. Molecular Orbital formed by subtraction of overlapping of two s orbitals. It is represented by s* (*) is used to represent antibonding molecular orbital) called Sigma Antibonding. Therefore, Combination of two atomic orbitals results in formation of two molecular orbitals, bonding molecular orbital (BMO) whereas other is anti-bonding molecular orbital (ABMO).
Q-Draw the energy level diagram of H2 molecule.
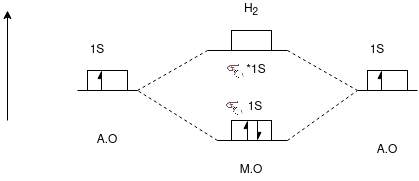
Q- Discuss about Carbon mono oxide with its energy level diagram, stabilization energy.
CO is the hetronuclear diatomic molecule. This molecule is formed by the combination of carbon and oxygen atom. Electronic configuration of carbon atom is 1s2, 2s2, 2p2 and that of oxygen is 1s2, 2s2, 2p4

Stabilisation Energy
[No. Of electron in BMO * (-) + No. Of electrons in anti bonding molecular orbital * (+)]
=(8) * (-) + (2) * (+)
= -6
Q-Explain N2 molecule.
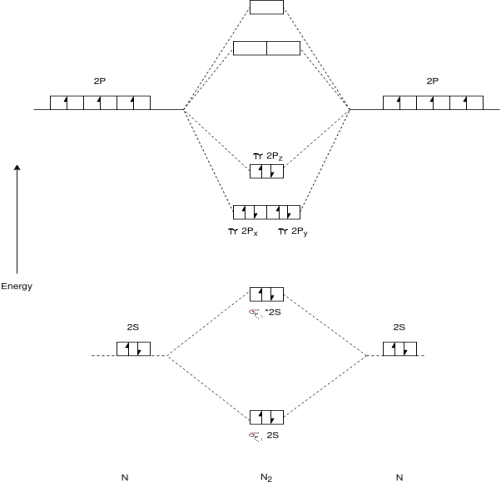
Q- Explain F2 molecule with its electronic configuration.

Q-Discuss about coordination chemistry.
The chemical structures in which a central metal atom is surrounded by nonmetal atoms or groups of atoms, called ligands, joined to it by chemical bonds. Coordination compounds include such substances as vitamin B12, hemoglobin, and chlorophyll, dyes and pigments, and catalysts used in preparing organic substances. A major application of coordination compounds is their use as catalysts, which serve to alter the rate of chemical reactions. Certain complex metal catalysts, E.g.:, they play a key role in the production of polyethylene and polypropylene. In addition, a very stable class of organ metallic coordination compounds has provided impetus to the development of organo metallic chemistry. Organ metallic coordination compounds are sometimes characterized by “sandwich” structures, in which two molecules of an unsaturated cyclic hydrocarbon, which lacks one or more hydrogen atoms, bond on either side of a metal atom. This results in a highly stable aromatic system.
Q-Write the nomenclature and IUPAC name of some compounds.
Common Name | IUPAC Name | Formula | Structure |
Acetylacetonato | 2,4-pentanediono | CH3COCHCOCH3 | 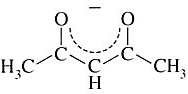 |
2,2-bipyridine | 2,2-bipyridine | Bipy | 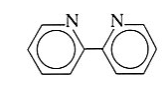 |
1,10-phenanthroline | 1,10-diaminophinanthrene | C12H8N2 | 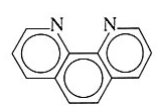
|
Dialkyldithiocarbamato | Dialklylcarbamodithioato | S2CNR2 | 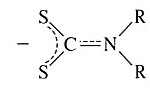 |
Oxalate | Oxalate | C2O4 | 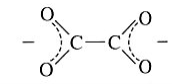 |
Dimethylglyoximato | Butanediene dioxime | HONCC(CH3)C(CH3)NO | 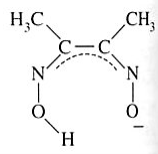 |
Q-Discuss about the spectra d-d transition.

 Many transition metal complexes show absorptions at relatively lower energies for electronic transitions (in the visible part of the spectrum). These are associated with transitions between d orbitals whose degeneracy has been removed by an environment of lower than spherical symmetry. E.g, in a octahedral complex, one may see transitions involving electron jumps from the t2g orbitals to the eg orbitals. These Orbitals are often only weakly bonding or antibonding - their energy separation is small. We may use the absorption energies to measure the ligand field splitting energies , but it should be noted that only in a few cases does the absorption energy correspond exactly to .
Many transition metal complexes show absorptions at relatively lower energies for electronic transitions (in the visible part of the spectrum). These are associated with transitions between d orbitals whose degeneracy has been removed by an environment of lower than spherical symmetry. E.g, in a octahedral complex, one may see transitions involving electron jumps from the t2g orbitals to the eg orbitals. These Orbitals are often only weakly bonding or antibonding - their energy separation is small. We may use the absorption energies to measure the ligand field splitting energies , but it should be noted that only in a few cases does the absorption energy correspond exactly to .
The study in which the transition metal complex ion absorbs electromagnetic radiation is called as the electron absorption spectroscopy. The absorbed radiation promotes electrons form the lower energy d-orbital to higher energy d-orbital. Such electronic transition are of high energy and in addition much lower energy vibrational and rotational transitions always occur. The vibrational and rotational levels are too close in energy. Therefore absorption bands due to high vibrational and rotational transitions are responsible for broadening of the electronic absorption in d-d spectra. Due to the promotion of electrons there occurs a change in the overall arrangement of electrons in d-orbital. The change in the overall arrangement of electrons in d-orbital gives rise to electron absorption spectrum.
The study of transition metal complex spectrum gives following:
(i) Color of complex
(ii) Magnitude of energy gap between ground state and excited state.
(iii) Extent of covalent character in metal ligand bond.
(iv) Geometry of the complex
The position of metal ions and ligand in spectrochemical series and nephelauxetic series.
Q-What is atomic spectroscopy?
Atomic spectroscopy deals with the consequences of inter electronic repulsion and magnetic interactions between electrons of an atom or an ion. These interactions involve the coupling of a spin and angular momentum of electrons within a given electronic configuration. The coupling rises several degenerate and non degenerate energy levels these energy levels are known as the energy terms or spectroscopic states. The electronic transition in these energy levels gives rise to electronic absorption spectrum.
Q- How to measure the electronic spectra of transition metal complexes?
The spectrum of a colored solution may be measured quite easily using a spectrophotometer. The beam of monochromatic light is passed through a solution and on to a photoelectric cell. The amount of light absorbed at any particular frequency can be read off or whole frequency range can be scanned. The absorbance If Io is the intensity of the original beam of light and I is the intensity after passing through the solution, then
A=log(Io/I)
The molar absorption coefficient is given by
E=A/Cl
Where,
C=concentration of solution
L= length