Unit-3
Physical, chemical and bacteriological examination of
water and wastewater
Q1) What Contaminants May Be in Our Supply?
A1) Safe Drinking Water Act (SDWA) has a very broad definition of “contaminant”, which includes literally all things that aren’t hydrogen or oxygen molecules. That being the case doesn’t let the word “contaminant” instantly strike fear into your heart. Many minerals that are found in your water cause no negative impacts on your health at all.
Some of these dissolved solids can cause hard water problems. Minerals like magnesium, iron, and calcium can cause issues with residue, rust, and soap scum build-up that can cause drains and wreak havoc on your skin, hair, and home appliances.
While annoying, 80% of the minerals and contaminants in your water are safe to consume. Other H2O invaders are less benign. The Water Quality Association has compiled a list of common water contaminants and their possible impacts on your home and health.
Contaminants can take many forms. Some of the most common include:
Physical – any material that changes the physical property of water, from sediment and dirt, to dissolved rock and other solids.
Chemical – some of these are natural, while others are the result of human impact.
Biological – parasites, bacteria, viruses, and other microorganisms.
Radiological – uranium, plutonium, and other elements that cause radioactive properties.
Q2) How do you determine the pH of a water sample?
A2) pH is a measure of how acidic or basic (alkaline) the water is (the term pH comes from the French: "puissance d'Hydrogène" which means strength of the hydrogen). It is defined as the negative log of the hydrogen ion concentration.
Stir the water sample vigorously using a clean glass stirring rod.
Q3) How would nitrogen content in water affect quality of water?
A3)
Nitrogen compounds with environmental relevance frequently analyted in wastewater are ammonia, nitrite, nitrate, and Kjeldahl nitrogen. Ammonia discharged to surface water can be nitrified in the aqueous environment if nitrifying microorganisms are present. The nitrifying bacteria consume dissolved oxygen for this process, thus depleting the oxygen content of the surface water with the consequence of massive dying of fish. Moreover, if the pH of the surface water is in the alkaline range, NH3 is formed which is toxic towards fish. The nitrate ion represents a nutrient leading to eutrophication of surface water, and nitrite is toxic and can react with amines (formed e.g. from amino acids of proteins) to yield N-nitrosamines which represent powerful carcinogens. Kjeldahl nitrogen is a sum parameter of compounds containing the nitrogen atom with an oxidation number of -3 (ammonia, amines and many other organic nitrogen compounds). It thus comprises organic nitrogen compounds besides ammonia nitrogen. This is also an important nitrogen parameter; because organic nitrogen compounds can be metabolized to ammonia (this conversion can also take place in surface water).
As many wastewater analyses (not only for nitrogen compounds) are photometric procedures, short information about photometry will be given. Photometry uses light as an analytical tool. As particular substances (analyses) absorb photons of different wavelengths to different extents, the wavelength (or colour) of the light applied for photometric analysis affects the specificity of the analytical procedure for a given analyte. The specificity can be increased by converting the analyte by reaction with certain reagents to form coloured products, because (besides the colour) also the reaction with a given reagent is specific for the analyte (other wastewater constituents would not react at all with the reagents used for conversion of a particular analyte). For example, ammonia can be converted to an intensely blue indophenols derivative by the following reactions:
NH4 + + OH-→ NH3 + H2O
NH3 + OCl-→ NH2Cl + OH-
NH2Cl + Phenol → Indophenol (intensely blue)
The last reaction is catalyzed by Mn2+ ions. For obtaining the blue product, an aliquot of the wastewater sample is mixed with a small volume of aqueous MnSO4 solution. Then the mixture is stirred and hypochlorous acid reagent and finally an alkaline aqueous phenol solution ("phenate reagent") are added. After 10 min the colour formation is complete for these particular reactions. The coloured product exhibits a maximum absorption at 630 nm (the complementary light causes the blue colour). The solution is transferred to a cuvette which is irradiated with light exhibiting a wavelength of 630 nm (satisfactory results are obtained in the 600 to 660 nm regions for this analytical procedure) and an intensity of Io in a photometer. In the photometer, the intensity of the light entering (Io) as well as the light leaving the cuvette (I) is determined (by means of a photodiode or a photomultiplier) as shown schematically in figure 6. The absorbance, i.e., log (Io/I), is linearly related to the Indophenol concentration as given by the Beer Lambert law:
Absorbance = log (Io/I) = 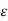 cd
cd
With the proportionality constant 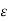 (molar absorptive or molar extinction coefficient), the length d of the way of the light through the cuvette (frequently 1 cm) and the molar concentration c of the coloured substance, resp. the concentration of the analyte in the sample (as one molecule of ammonia will yield one molecule of the coloured substance, the absorbance will also be linearly related to the ammonia concentration in the wastewater or in calibration solutions, resp.).
(molar absorptive or molar extinction coefficient), the length d of the way of the light through the cuvette (frequently 1 cm) and the molar concentration c of the coloured substance, resp. the concentration of the analyte in the sample (as one molecule of ammonia will yield one molecule of the coloured substance, the absorbance will also be linearly related to the ammonia concentration in the wastewater or in calibration solutions, resp.).
The colourless nitrite ion NO2- is also transformed to a coloured substance prior to photometric analysis. A standard method used for nitrite analysis suitable for determinations down to 1 µg NO2-N/l is the reaction of nitrite at pH 2 (formation of nitrous acid) with sulfanilic acid to give a diazonium salt which reacts with another reagent, (1-naphthyl)-ethylenediamine, in order to form a reddish-purple azo dye that can be detected photometrically at 543 nm.
As for other analytes, also for nitrate determination several analytical methods can be applied.
Q4) How would toxic metals and its compounds affect quality of water?
A4) For the determination of metals, there exist special methods as flame emission photometry (e.g., important for the fertilizer component potassium). In this procedure the aqueous sample is transferred into a flame where the metals are electronically excited resulting in an emission of light of a particular wavelength. This emission can be detected and used for quantification of the concerning metal ion.
A similar method is also useful for the determination of some toxic heavy metals (atomic emission spectrometry/inductively coupled plasma, AES/ICP). The aqueous solution is pumped into a small plasma generated by high frequency fields where the metals are electronically excited leading to emission of light of that wavelength which is characteristic for the particular metal of concern. With this method, several metals can be determined simultaneously.
On the other hand, aqueous solutions of metal salts can also absorb distinct wavelengths of light, when they are heated to very high temperatures (flame or graphite furnace) and converted from ions to atoms by this. The light absorbed by the atoms can be used for quantification of particular metal ions in aqueous solutions like wastewaters. The method is called atomic absorption spectrometry (AAS). Solids have to be digested prior to AAS analysis if their metal content is to be analyzed. Details for such methods can be read in the "Standard Methods".
Sometimes, there is interest in the concentrations of particular organic compounds contained in wastewaters. For such analyses, gas chromatography is a useful tool, but very complex in execution. For many gas chromatographic methods, wastewater samples have to limits for particular trace organics. And the final concentrate is then analyzed. A very small volume of the concentrate (in the range of one µl) is transferred to the so-called injector of the gas chromatograph by a syringe. The injector is heated to temperatures in the range of 200°C and flushed by t he inert carrier gas (very often helium is used). At these high temperatures the total solution evaporates at once and the analytes as well as the extractant are transported by the carrier gas to a separation device, the so-called column. The column is usually a capillary made of fused silica (a material that has substituted glass which had been used earlier for manufacturing capillaries for gas chromatography) of some 10 m length.
Q5) If the BOD of a municipal wastewater at the end of 7 days is 60.0 mg/L and the ultimate BOD is 85.0 mg/L, what is the rate constant?
Given: 7-day BOD = 60.0; L = 85.0 mg/L.
A5)
BODt = Lo (1 – e-kt)
60.0 = 85.0 (1 – e-kt)
0.7059 = 1 – e-kt
-0.2941 = - e-kt
Divide through by -1 and take ln of both sides
ln (0.2941) = ln e-kt
-1.224 = -k (7)
k = 0.1748 d-1
Q6) Calculate the amount of 1 N sodium hydroxide or 1 N sulfuric acid needed to neutralize the sample to pH 7.0 using the following formula:
A6)
mL needed = (mL acid or base used x mL total test sample)/mL sample portion used for neutralization.
Suppose 1.3 mL of 1 N NaOH is used to neutralize 50 mL of sample to pH 7.0. Calculate the volume of NaOH to be added to neutralize the sample as follows:
mL 1 N NaOH needed = (1.3 mL x 1000 mL)/50 mL
= 1300/50 = 26 mL
Q7) A 25 ml of sewage water sample was refluxed with 10 ml 0f 0.25 N K2Cr2 O7 solution. The untreated dichromate requires 6.5 ml of 0.1 N FAS.10 ml of dichromate solution and 25 ml distilled water, under the same condition as sample required 27 ml of 0.1 n FAS. Calculate the COD of sewage.
A7)
COD = V2 –V1 X N X 8 X 1000/V
V2 = volume of FAS for blank titration
V1 = volume of FAS for sample titration
V = volume of sample taken for test
N = Normality of FAS
COD = 27 –6.5 X 0.1X 8 X 1000/25
COD = 656 ppm
Q8) What are the water quality standard in India?
A8)
Characteristics | Designated best use | ||||
A | B | C | D | E | |
Dissolved Oxygen (DO)mg/l, min | 6 | 5 | 4 | 4 | - |
Biochemical Oxygen demand (BOD)mg/l, max | 2 | 3 | 3 | - | - |
Total coliform organisms MPN/100ml, max | 50 | 500 | 5,000 | - | - |
pH value | 6.5-8.5 | 6.5-8.5 | 6.0-9.0 | 6.5-8.5 | 6.0-8.5 |
Colour, Hazen units, max. | 10 | 300 | 300 | - | - |
Odour | Un-objectionable |
| - | - | |
Taste | Tasteless | - | - | - | - |
Total dissolved solids, mg/l, and max. | 500 | - | 1,500 | - | 2,100 |
Total hardness (as CaCO3), mg/l, and max. | 200 | - | - | - | - |
Calcium hardness (as CaCO3), mg/l, max. | 200 | - | - | - | - |
Magnesium hardness (as CaCO3), mg/l, max. | 200 | - | - | - | - |
Copper (as Cu), mg/l, max. | 1.5 | - | 1.5 | - | - |
Iron (as Fe), mg/l, max. | 0.3 | - | 0.5 | - | - |
Manganese (as Mn), mg/l, max. | 0.5 | - | - | - | - |
Chlorides (as Cu), mg/l, max. | 250 | - | 600 | - | 600 |
Sulphates (as SO4), mg/l, max. | 400 | - | 400 | - | 1,000 |
Nitrates (as NO3), mg/l, max. | 20 | - | 50 | - | - |
Fluorides (as F), mg/l, max. | 1.5 | 1.5 | 1.5 | - | - |
Phenolic compounds (as C2H5OH), mg/l, max. | 0.002 | 0.005 | 0.005 | - | - |
Mercury (as Hg), mg/l, max. | 0.001 | - | - | - | - |
Cadmium (as Cd), mg/l, max. | 0.01 | - | 0.01 | - | - |
Selenium (as Se), mg/l, max. | 0.01 | - | 0.05 | - | - |
Arsenic (as As), mg/l, max. | 0.05 | 0.2 | 0.2 | - | - |
Cyanide (as Pb), mg/l, max. | 0.05 | 0.05 | 0.05 | - | - |
Lead (as Pb), mg/l, max. | 0.1 | - | 0.1 | - | - |
Zinc (as Zn), mg/l, max. | 15 | - | 15 | - | - |
Chromium (as Cr6+), mg/l, max. | 0.05 | - | 0.05 | - | - |
Anionic detergents (as MBAS), mg/l, max. | 0.2 | 1 | 1 | - | - |
Barium (as Ba), mg/l, max. | 1 | - | - | - | - |
Free Ammonia (as N), mg/l, max | - | - | - | 1.2 | - |
Electrical conductivity, micromhos/cm, max | - | - | - | - | 2,250 |
Sodium absorption ratio, max | - | - | - | - | 26 |
Q9) Briefly explain the technique known as disposal by dilution.
A9)
In this process, the raw sewage or the partially treated sewage is thrown into natural waters having large volume. The sewage in due course of time is purified by what is known as the self-purification capacity of natural waters. The limit of discharge and degree of treatment of sewage are determined by the capacity of self-purification of natural waters.
Conditions favorable for dilution
Following conditions are favorable for sewage to be disposed of by dilution into natural waters
Q10) How the wastewater gets disposed of by disposal by land treatment?
A10)
Here, the raw domestic waste water (sewage) is applied on the land. A part of sewage evaporates and the remaining portion percolates through the ground and is caught by the underground drains for disposal into natural waters. The sewage adds to the fertilizing value of land and crops can be profitably raised on such land. The term sewage farming is also sometimes used for indicating disposal of sewage by land treatment. The design of a good land treatment system demands the services of environmental engineers, hydraulic engineers, irrigation engineers, agronomists, soil scientist, etc.
Conditions favorable for land treatment
Preventive measures
In order to prevent sewage sickness of land, the following preventive measures may be adopted
1. Alternative arrangement: There should be ample provision of extra land so that land with sewage sickness can be given the desired rest. Alternatively, sewage should be disposed of by some other method when sewage farms are taking rest
2. Depth of sewage: If sewage is applied in excess, the chances of sewage sickness are increased. The land is unable to receive the excess sewage in a satisfactory way and it ultimately clogs up. Depth of sewage on land should be carefully decided by keeping in view the climatic conditions, drainage facilities, nature of crops and characteristics of soil.
3. Drainage of soil: Subsoil drain pipes should be laid in sufficient number to collect the percolated effluent
4. Intermittent application: Sewage should be applied on land at intervals. The period between successive applications depends on general working of sewage farm and the permeability of soil. Depending on the nature of the soil, this period between successive applications varies from few hours to few weeks.
5. Pretreatment of sewage: sewage should be given some pretreatment before it is applied on land.
6. Rotation of crops: It is desirable to grow different types of crops on a piece of land instead of one single crop. Rotation of crops minimizes the chances of sewage sickness.
7. Treatment to land: The land affected by sewage sickness should be properly treated before it is put up in use again. Clogged surfaces should be broken by suitable equipment.