Module 5
Polymer
Q1-Discuss about the different type of Polymer-Blend.
A- Miscible polymer blend: Polymer blend, homogenous down to the molecular level, associated with the negative value of the free energy of 0, and a positive value of the second derivative: ΔHmmixing: ΔGm ∂ 2ΔGm/∂φ2 > 0. It is a single –phase structure , has properties of average values between the values of properties of its components and has one Tg.
Immiscible polymer blend: A blend exhibits more than two phases. It ΔHmis a blend whose free energy of mixing; ΔGm > 0 Usually, has two Tg's , since the two components are phase separated. Miscible PB Immiscible PB Scientists often measure the Tg of a blend to find out if it is miscible or immiscible. If two Tgs are found, then the blend is immiscible. If only one Tg is observed , then the blend is likely to be miscible.
Homologous polymer blend: Mixture of two or more fractions of the same polymer each of which has different molecular weight distribution.
Isomorphic polymer blend: Polymer blend of two or more different semi-crystalline polymers are miscible in the crystalline state as well as in the molten state.
Compatible polymer blend: An immiscible blend is called compatible if it is useful blend wherein the inhomogeneity (caused by different phases) is on a small enough scale not to be apparent in use.
Q2- What do you understand by biodegradable polymer?
A- It is a processes of converting polymer material into harmless simple gaseous products by the action on enzymes of microorganism and water.
Q3-Explain PHBV?
A-
It is a co – polymer of hydroxy butyric acid and 3 hydroxyvaleric acid. It is produced by fermentation of glucose by acaligeneseutrophus species.
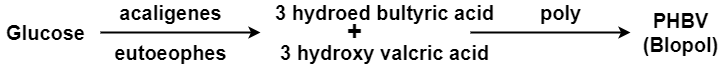
Properties: -
Limitations: -
Applications: -
Q4- Explain the application of n type doping.
A-
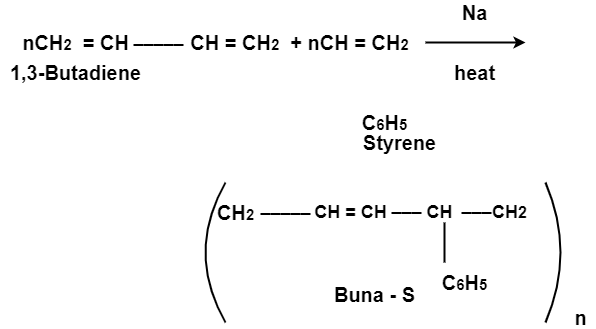
Q5- Explain the preparations of Buna-S.
A- Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrine. Buna-S is the replacement of natural rubber while styrene, 2 monomers and butadiene play a major role in its derivation where as these 2 monomers is polymerized by two basically different process i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene.
It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called as cold rubber. The obtained rubber is called as the Styrene Butadiene Rubber (SBR).
Q6- Applications of Neoprene.
A-
(i) They are used as the base for the adhesives, noise isolation in power transformations.
(ii) The burning point of neoprene is about 260o C.
(iii) They are used as the load bearing base.
(iv) They used in home accessories.
(v) Some experts evaluated that neoprene can be helpful in making the home based mask.
Q7-Explain the preparation of Nylon-6.
A- Nylon 6 is also called as the Polycaprolactam which is developed by Paul Schlack. They are semicrystalline polyamide.They have smooth surface and are featureless as a glass rods. Nylon 6 can be modified using comonomers or stabilizers during polymerization to introduce new chain end or functional groups, which changes the reactivity and chemical properties. It's often done to change its dyeability or flame retardance. Nylon 6 is synthesized by ring-opening polymerization of caprolactam. When caprolactam is heated at about 533 K in an inert atmosphere of nitrogen for about 4–5 hours, the ring breaks and undergoes polymerization. Then the molten mass is passed through spinnerets to form fibres of nylon 6.
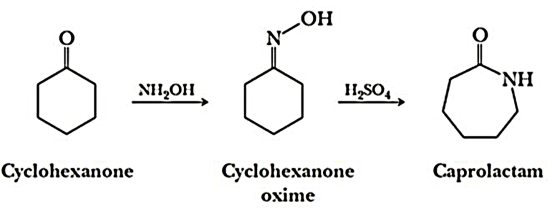
On further heating of Caprolactam at 533K with inert atmosphere of nitrogen for about 4-5 hours it gives
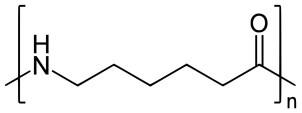
(Nylon-6)
Q8- Explain the preparation Terylene with its applications.
A- Terylene is a polyester fibre formed by the polymerisation of dimethyl terephthalate with ethylene glycol. Dimethyl terephthalate is heated with ethylene glycol at 503 K in the presence of zinc acetate and antimony trioxide as a catalyst when dihydroxydiethyl terephthalate (monomer) is formed, Dihyd- roxydiethyl terephthalate on heating polymerises to give terylene.
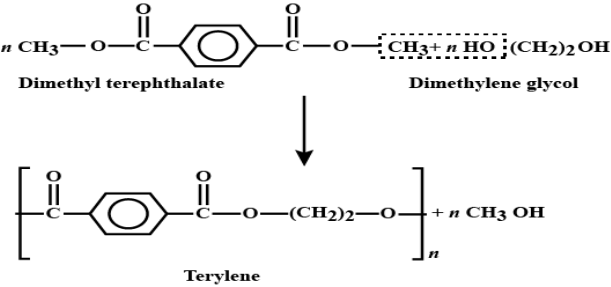
Applications:
(i) Terylene are very strong fiber and suffer very little loss in strength when wet.
(ii) They are elastic in nature that is why they possess the property of resist creasing.
(iii) It can be set into permanent pleats when subjected to the correct temperature at time of manufacturing.
(iv) They are easily washable and quickly dry.
Q9- Complete the following reaction
(i)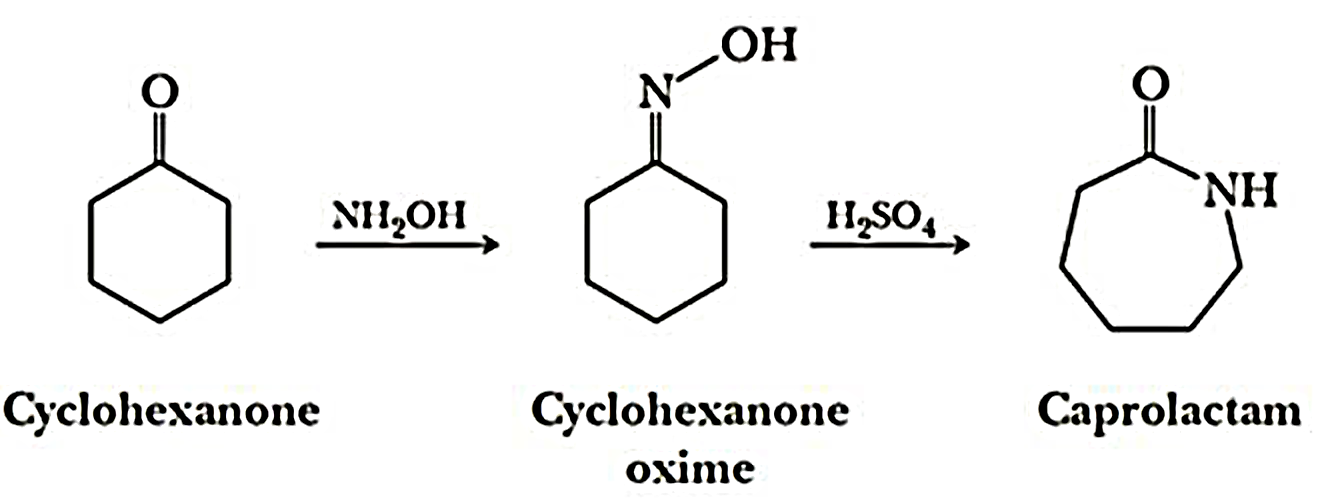
(ii)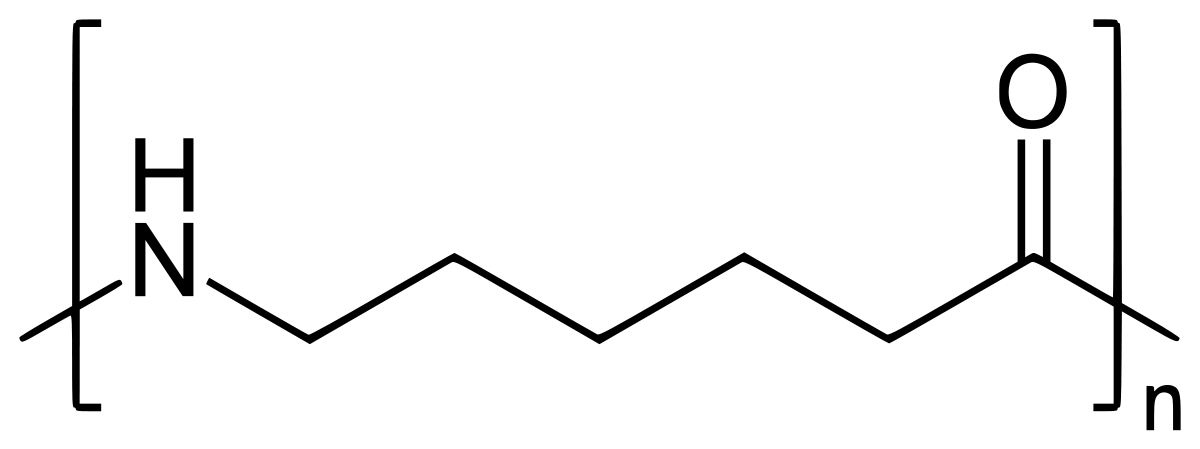
(iii)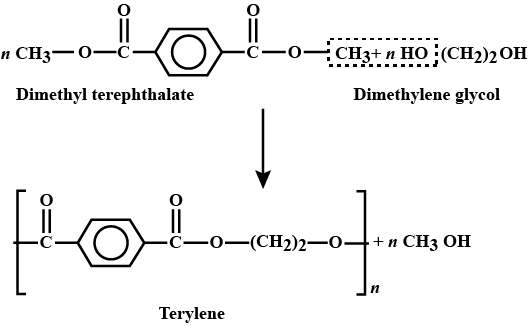
(v) 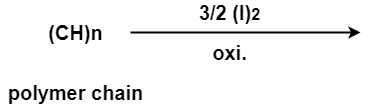
A- (i) 
(ii)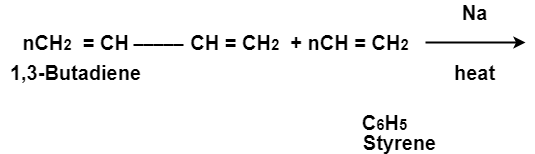
(iii)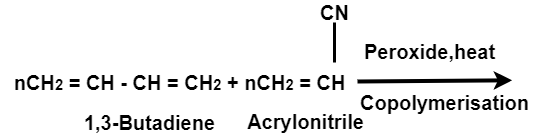
(iv) 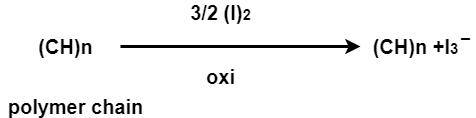
Q10- Explain Grignard reaction.
A-
Grignard reagent is widely is used as organmetallic reagents. They are prepared in ethereal solution by the reaction of organic halide with magnesium.
 RX + Mg RMgX
RX + Mg RMgX
Where, X are halides i.e., Chlorine, bromine, Iodine
They are quite reactive in general while stable in ethereal solution. Where as the organometallic reagents are those compounds which contains carbon-metal bonds.

The compound attached to the organic compound may be the Li or Mg i.e., organolithium reagent while in the presence of Magnesium this is called as the Grignard Reagent. The Grignard Reagent play as the most versatile source of nucleophilic carbon. The nucleophilic character of organometallic reagents stems from the fact that the C-M bond is polarized in such a way that the carbon atom is negative while the metal atom is positive.