Unit – 3
Vapour Absorption system
Q1) Explain the working Principal of vapour absorption refrigeration system.
A1)
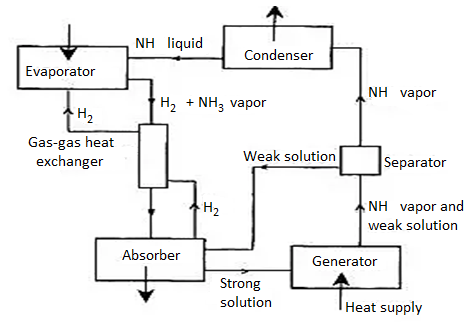
Working Principal of vapour absorption refrigeration system:
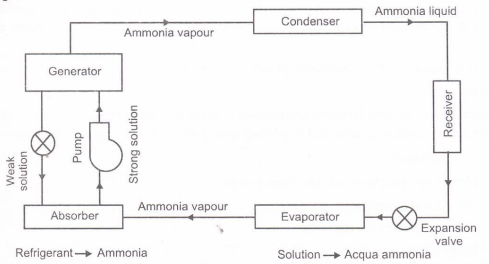
Fig. Simple vapour absorption system
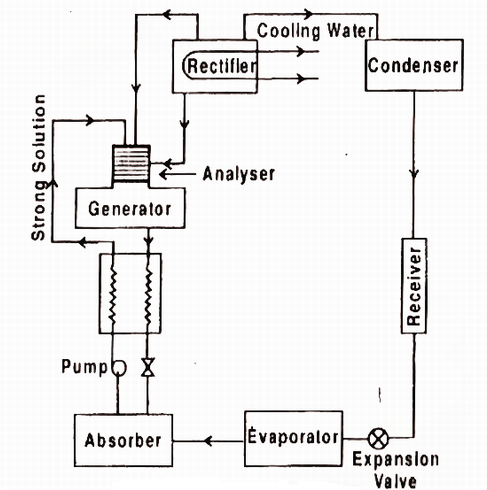
Fig. Practical Absorption system
Principle of Vapour Absorption System:
- There is the peculiar property of some substances to have affinity for other substances at some temperature and pressure conditions and less affinity at another conditions.
- This idea for the working principle of a vapour absorption system was generated by Michael Faraday in 1824.
- He knew that silver chloride (AgCl) a white powder, had a property of absorbing large amount of ammonia gas at the normal temperature and pressure.
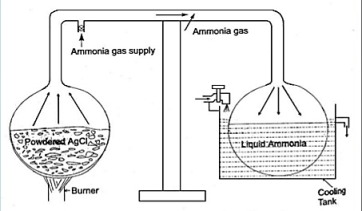
Fig. Intermittent vapour system
- Two chambers are combined with the help of a tube.
- The white powder was kept inside the first chamber to which ammonia gas was supplied and sealed.
- The powder was heated up while other end was cooled using circulating water.
- Liquid ammonia was obtained in the cool end of the apparatus. After stopping heat, it was observed that, the liquid ammonia instead of sitting there, started boiling (bubbles produced) and vapour was reabsorbed by the white powder.
- Upon touching the boiling end, it was astonished to find that the vessel was very cold.
- He repeated the experiments and cooling was observed again.
- This led to invention of the intermittent
Q2) Write the Comparison between absorption & compression systems.
A2)
Comparison between absorption & compression systems are as follows:
Vapour Absorption system | Vapour Compression system |
Uses low grade energy like heat. Therefore, may be worked on exhaust system from I.C Engine, etc. | Uses high grade energy like mechanical work. |
Moving parts are only in the pump, which is a small element of the system. Hence operation is smooth. | Moving parts are in the compressor. Therefore, more tear, wear and noise. |
The system can work on lower evaporator pressure also without affecting COP. | The COP decreases considerably with decrease in evaporator pressure. |
No effect of reducing the load on the performance. | Performance is adversely affected at partial loads. |
Liquid traces of refrigerant present in piping at the exit of evaporator. | Liquid traces in suction line may damage the compressor. |
Q3) Explain the Lithium- Bromide water vapour absorption system.
A3)
Lithium- Bromide water vapour absorption system
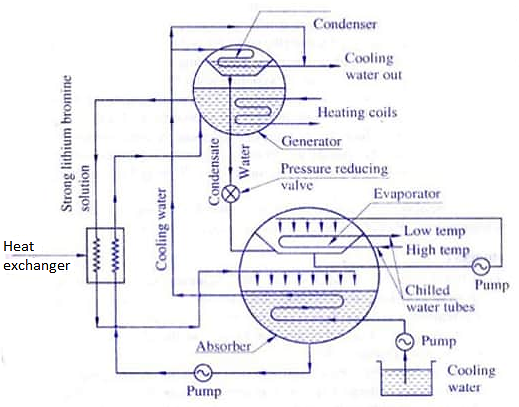
LiBr is non-toxic, readily available and inexpensive. This solution is confined to the absorber and generator sections of the cycle. It is used to move the refrigerant (distilled water) from low pressure to high pressure.
The absorbent (LiBr) should have a strong affinity to absorb the refrigerant (H2O). The solution of absorbent and refrigerant should have a boiling point that is much higher than the refrigerant (water) alone. This makes it easy to separate the absorbent from the refrigerant at high temperatures. A pump is used to circulate the absorbent-refrigerant solution between the absorber and generator. The pump also increases the pressure.
Another common refrigerant-absorbent combination is ammonia as the refrigerant and water as the absorbent. This combination is sometimes used in small residential application. This discussion assumes the refrigerant is Distilled Water (H2O) and the absorbent is Lithium Bromide (LiBr).
The two fluids, refrigerant and absorbent, are mixed inside the chiller in various concentrations. The term “dilute solution” refers to a mixture that is high in refrigerant (H2)) and low in absorbent (LiBr). “Concentrated solution” refers to a mixture that is low in H2O and high in LiBr. “Intermediate solution” is somewhere in between.
The components of the absorption refrigeration cycle are the evaporator-absorber on the low-pressure side and the generator-condenser on the high-pressure side. The pressure on the high-pressure side is about ten times the low-pressure side.
Q4) Write the advantages and disadvantages of Lithium- Bromide water vapour absorption system.
A4)
Advantages:
• Feasible to separate refrigerant from absorbent, as water is more volatile compared to Li-Br.
• Operating pressures are low so pumping cost is low.
• Wall thickness of the system less compared to VCR.
• Water as refrigerant is nontoxic, so can be directly used for chilled coil.
• Can be built up to 1,00,000 TR capacity plants.
• Operation and maintenance cost is very low.
Disadvantages:
• Evaporation temperature must be kept above the freezing point of water and hence the temperature of chilled water cannot be less than 5oC.
• Li-Br solution is corrosive. So, inhibitors need to be added to the system to prevent the metal parts from corrosion.
Q5) What do you mean by three-fluid refrigeration system.
A5)
The three-fluid refrigeration system has three fluids as follows:
- Ammonia
- Water
- Hydrogen
This is basically vapour absorption refrigeration system and here ammonia is refrigerant and water is absorbent. Hydrogen gas circulated in evaporator and absorber to increase the rate of evaporation of ammonia in evaporator.
Three fluid refrigeration is a system which does not needs any mechanical work or electrical works (no pump or compressor).
This was found by two students from Sweden.
This is designed to overcome the drawbacks of conventional two fluid VAR systems. There we will use a pump to pump the solution from absorber to the generator.
This system has Hydrogen as third fluid along with Ammonia(refrigerant), Water(absorber).
Q6) What do you mean by Refrigerants and classify them.
A6)
Refrigerants:
The transfer of heat from a low-temperature region to a high-temperature one requires special devices called refrigerators.
The objective of a refrigerator is to remove heat (QL) from the cold medium; the objective of a heat pump is to supply heat (QH) to a warm medium.
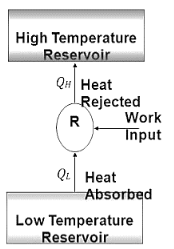
Device used to maintain low temperature below atmospheric temperature within required space.
‘Method of reducing the temperature of a system below surrounding temperature and maintains it at lower temperature by continuously abstracting heat from it.’
Classification of refrigerants
Refrigerants are classified as follows:
- Primary refrigerants are those working mediums or heat carries which directly take part in refrigeration system and cool the substance by the absorption of latent heat e.g., Ammonia, Carbon dioxide, Methyl chloride etc.
- Secondary refrigerants are those circulating substances which are first cooled with the help of the primary refrigerants and are then employed for cooling purposes, e.g., ice, Carbon dioxide etc. These refrigerant cool substances by absorption of their sensible heat.
Q7) Explain the Designation of Refrigerants.
A7)
Designation of Refrigerants: - The international designation committee of refrigerants uses Refrigerant or R as the designation followed by certain numbers (e.g., R-21, R-40, R-30, R-744 etc.)
A refrigerant followed by a two-digit number indicates that the refrigerant is derived methane base while a three-digit number represents ethane base.
The general chemical formula for a compound derived from a saturated hydrocarbon is given by as: CaHbFcCld
Where, b + c + d=2a+2, and,
a= Number of carbon atoms,
b= Number of hydrogen atoms,
c= Number of fluorine atoms,
d= Number of chlorine atoms, the complete designation of the refrigerant is given by: R (a-1) (b+1) (c)
Example: In case of Dichlorodifluoromethane (CCl2F2):
a=1, b=0, c=2, d=2
So, the designation is: R (1-1) (0+1) (2) i.e., R-12.
Q8) Name the Refrigerants which is used in refrigeration systems.
A8)
A refrigerant is a substance used for refrigeration. The best refrigerant has good thermodynamic properties, is chemically non-reactive, and is safe. Because some refrigerants can cause severe damage to the ozone layer, it was decided in 1992 to make it illegal to release refrigerants into the atmosphere.
Refrigerants used in refrigeration systems are as follows:
Refrigerant R11: R11 is a CFC refrigerant, which means it is made of chlorine, fluorine, and carbon. R11 is typically used in the refrigerators found in office building and hotel air conditioning systems because it allows large refrigerators to cool large amounts of water at low costs. In the past, when air would leak into R11 systems, that air had to be purged, and usually some of the refrigerant would be lost. Through newer technological advances and better maintenance, less R11 has been lost in these large refrigerators. In view of the global environmental problem resulting from global warming, depletion of the ozone layer, this CFC refrigerant is currently being pursued internationally.
Refrigerant R22: R22 belongs to the HCFC group of refrigerants, which means it's made of hydrogen, chlorine, fluorine, and carbon. R22 is the most common refrigerant on the market as it is used in most residential and commercial air conditioning systems and even in some large centrifugal refrigerators. R22 is also pursued internationally for its GWP (Global warming potential) and ODP (Ozone depletion potential).
Although it is a popular refrigerant, it will be phased out in new refrigeration equipment that is made in 2010, and it will stop being produced in 2020.
Refrigerant R422B: R422B is a refrigerant made by ICOR to be similar to the R22 refrigerant. Like the R22 it is made for residential and commercial air conditioners. R422B is an HFC refrigerant, which means that it is made of hydrogen, fluorine, and carbon. This hydrogen and carbon in refrigerant help oil return in those refrigeration systems that have mineral oil or alkyl benzene in them. R422B won't mix with these oils, but the hydrogen and carbon allow the oil to thin out and keep moving in these systems.
Refrigerant R717: R717 is the refrigerant free from any halogen atoms. It is named as ammonia. The ammonia–water absorption refrigerator has been used widely in refrigeration and air-conditioning applications. R717 has a wide range of applications. It is particularly suited to working in the range approximately 0°C to -30°C and hence is widely used for food preservation. This includes the chilling of liquids such as milk, beer and soft drinks, enlarge cold storage facilities, meat processing and packing plants, large ice-making plants and commercial refrigeration. Other common applications include large air conditioning systems (refrigerators), industrial heat extraction and ice rinks.
An advantage of using R717 is its zero-ozone depletion potential and zero global warming potential.
Refrigerant R718: R718 is nothing but water. Water can be used as a refrigerant in refrigerators without any safety measurement which is cheap, environmentally neutral. Its maintenance cost is very low since leakages can be accommodated from the system.
Q9) What are the Desirable properties of refrigerants.
A9)
Desirable properties of refrigerants
A refrigerant is said to be ideal if it has all of the following properties: -
1. Low boiling point and High critical temperature.
2. High latent heat of vaporization and Low specific heat of liquid.
3. Low specific heat of liquid and Low specific volume of vapour.
4. Low specific volume of vapour.
5. Non-corrosive to metal.
6. Non-flammable and non-explosive, non-toxic.
7. Low cost.
8. Easy to liquidate moderate pressure and temperature.
9. Easy of locating leaks by suitable indicator, and
10. Mixes well with oil.
Q10) Name few refrigerants which are available commercially in the market.
A10)
The refrigerants which are available commercially in the market are numerous. Some of them which are in common use are mentioned below:
- Air: Air (molecular weight 28.97, specific heats cp = 1.04 kJ/kgK and cv = 0.712 kJ/kg-K) is one of the earliest refrigerants to be used in the refrigeration systems. Its advantages are that it is available free of cost, is non-toxic and non-flammable and does not affect the commodity if pure. However, air suffers from a number of drawbacks. Air contains moisture and this reacts with the material of the evaporator and condenser severely affecting their working capacity. Further, there is a possibility that the passages may be blocked by the formation of ice from this moisture. The COP of air is of the order of 0.6 and thus, not suitable for use in refrigeration systems on a Refrigerants commercial scale. It is mainly used for air conditioning in aircrafts where efficiency of operation is of secondary importance.
- Ammonia: Ammonia (molecular weight 17) is one of the oldest refrigerants and it was commonly employed in places where toxicity effects were of secondary importance. Its advantages are its low cost, low specific volume, high COP (of the order of 4.0) and high refrigeration effect per unit mass of the refrigerant. Its primary drawback is its toxicity which prevents its use in air- conditioning and food preservation systems. Ammonia has a boiling point of -33 0 C at atmospheric pressure.
- Carbon Dioxide: Carbon dioxide (molecular weight 44) is a non-toxic and non-poisonous refrigerant. Also, it is not only non-flammable but and is an excellent extinguishing agent as well. Its other advantages are that it is chemically stable, immiscible with the lubricating oil and does not affect the metal used in the system. It has a low specific volume and this requires volume displacement per ton of refrigeration. However, its critical pressure is too high. Also, its critical temperature is only 31 0 C which makes it unsuitable for use in countries with a hot climate like India. It is an excellent refrigerant for low temperature refrigeration.
- Sulphur Dioxide: Sulphur dioxide (molecular weight 64) is a colourless, suffocating and irritating gas and is twice as heavy as air at atmospheric conditions. It was mostly used as a household refrigerant in the older days, but has since been discarded for better refrigerants. It suffers from a lot of disadvantages. Sulphur dioxide reacts with water forming sulphurous acid, which in presence of oxygen becomes sulphuric acid, a corrosive compound for metals. It is non-flammable but attacks foodstuff on coming in contact with it. It is also partially miscible with the lubricating oil.
- Hydrocarbons: This group consists of colourless fluids normally in gaseous state and made up of various combinations of carbon and hydrogen. Most of the refrigerants from this category are suitable for low temperature refrigeration. Isobutane falls in this category and has been suitable for domestic refrigeration. They are non-poisonous, but are flammable and highly explosive when exposed to air. The molecular weight and boiling point of each gas varies according to the number of hydrogen and carbon atoms. The larger the number of hydrogen and carbon atoms, the heavier is the gas and higher is its boiling point.
- Halocarbon Refrigerants: The halocarbon refrigerants are formed by replacing one or more of hydrogen atoms of methane or ethane by one or more atoms of the three halogens: fluorine, chlorine or bromine. Some of the refrigerants coming under this category are mentioned below:
- Refrigerant R12: The refrigerant R12 is the most widely used refrigerant in the domestic and large commercial establishments. Its chemical formula is CCl2F2 and its boiling point is -300 C at 1 bar. It is a non-flammable, non-explosive, non-irritating, non-toxic and odourless refrigerant.
- Refrigerant R13: Its chemical formula is CClF3. It is a non-flammable, non-toxic and stable refrigerant. It is very suitable for achieving low temperatures in a cascade refrigeration system. Its specific volume is high and therefore, it is suitable for centrifugal compressors. However, it also has a negative effect on ozone depletion.
- Refrigerant R22: Its chemical formula is CHClF2. It is also a non-toxic, non-flammable, non-corrosive and non-irritating refrigerant. It is the most common refrigerant for use in large refrigeration systems and is preferred to R12.
- Refrigerant R114: Its chemical formula is C2Cl2F4. Its boiling point corresponding to 1 bar is about 30 C. It has properties very similar to those of R12 with respect to water and oil combination. It is not suitable for low temperature refrigeration since it has negative evaporator pressure even at around 9 0 C. It is non-toxic, non-explosive and non-corrosive even in the presence of water.
Q11) Explain elementary idea of refrigerant absorbers
A11)
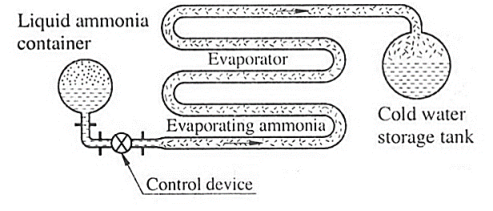
Fig. Liquid absorbent system
- Solubility requirement: The refrigerant should have proper solubility in the absorbent so that a strong solution, highly rich in the refrigerant, is formed in the absorber by the absorption of the refrigerant vapour.
- Boiling point requirement: There should be a large difference, about 200 degree C, in the boiling points of the two substances, thus absorbent free refrigerant is boiled off from the generator.
Some other Properties required:
- The refrigerant should have high affinity for the absorber at low temp and less affinity at high temp.
- It should have low freezing point.
- It should have good thermal and chemical stability
- Irreversible chemical reactions of all kinds are to be avoided.
Q12) Write note on nomenclature.
A12)
Designation of Refrigerants: - The international designation committee of refrigerants uses Refrigerant or R as the designation followed by certain numbers (e.g., R-21, R-40, R-30, R-744 etc.)
A refrigerant followed by a two-digit number indicates that the refrigerant is derived methane base while a three-digit number represents ethane base.
The general chemical formula for a compound derived from a saturated hydrocarbon is given by as: CaHbFcCld
Where, b + c + d=2a+2, and,
a= Number of carbon atoms,
b= Number of hydrogen atoms,
c= Number of fluorine atoms,
d= Number of chlorine atoms, the complete designation of the refrigerant is given by: R (a-1) (b+1) (c)
Example: In case of Dichlorodifluoromethane (CCl2F2): a=1, b=0, c=2, d=2 So, the designation is: R (1-1) (0+1) (2) i.e., R-12.
Few refrigerants and their use:
A refrigerant is a substance used for refrigeration. The best refrigerant has good thermodynamic properties, is chemically non-reactive, and is safe. Because some refrigerants can cause severe damage to the ozone layer, it was decided in 1992 to make it illegal to release refrigerants into the atmosphere.
Q13) What is secondary and environment friendly refrigerator.
A13)
Secondary refrigerants are those circulating substances which are first cooled with the help of the primary refrigerants and are then employed for cooling purposes, e.g., ice, Carbon dioxide etc. These refrigerant cool substances by absorption of their sensible heat.
In some refrigeration systems, the refrigerant is not put into direct use. This is done mainly out of safety considerations.
As an example, it is not desirable to use toxic refrigerants like ammonia in home air- conditioning and home refrigeration systems. Also, in some cases the size of the refrigerated space may be so large that direct refrigeration may be uneconomical.
In such case, an indirect way is employed. The refrigerants used in this way do not pass through the cyclic process and are referred to as secondary refrigerants. The refrigerants commonly used in this way are water and brine solutions of calcium or sodium.
This
1. UV radiation hits CFC molecule
2. Chlorine atom breaks away.
3. Chlorine atom hits ozone molecule.
4. Chlorine atom takes one oxygen atom to form chlorine monoxide and one molecule of oxygen.
5. Oxygen atom hits chlorine monoxide molecule.
6. Two oxygen atoms form an oxygen molecule. Chlorine atom is free and repeats the depletion process.
Q14) What is Phase changing materials.
A14)
- A section alternate cloth (PCM) is a substance which releases/absorbs enough strength at section transition to offer beneficial warmth/cooling.
- Generally the transition can be from one of the first essential states of matter - strong and liquid - to the different.
- The section transition will also be among non-classical states of matter, along with the conformity of crystals, in which the cloth is going from conforming to at least one crystalline shape to conforming to another, which can be a better or decrease strength state.
- The strength launched/absorbed via way of means of section transition from strong to liquid, or vice versa, the warmth of fusion is typically lots better than the practical warmth. Ice, for example, calls for 333.fifty five J/g to melt, however then water will upward push one diploma similarly with the addition of simply 4.18 J/g.
- Water/ice is consequently a completely beneficial section alternate cloth and has been used to shop iciness bloodless to chill homes in summer time season on account that as a minimum the time of the Achaemenid Empire.
Q15) Write short note on ozone layer depletion.
A15)
- An issue of growing concern for the present-day environment is the impact of the various refrigerants on the ozone depletion and global warming of the environment.
- The main culprits in this case are the chlorine containing halogenated hydrocarbons, commonly known as chlorofluorocarbons or CFC which are being used as refrigerants.
- The Earth’s atmosphere is made up of various layers. The layer just above the Earth’s surface is known as the troposphere.
- The troposphere extends up to 10 km from the surface. The ozone layer is just above the troposphere and located in the stratosphere.
- The stratospheric ozone is Earth’s natural protection to harmful ultraviolet (UV) radiation from the sun. UV radiation is harmful to human, plant and animal life.
- The ozone layer gets depleted by the action of these refrigerants. CFCs, when they are released from the surface of the Earth, rise slowly into the stratosphere.
- Here they are bombarded by the incoming UV light from the Sun, which releases the chlorine atoms from the parent compound. It is this chlorine atom which reacts with the ozone molecules.