Unit-1
Polymer Science
Q1)What is a polymer?
A1)
Polymer chemists’ study large, complex molecules that are built up from many smaller units. They study about the smaller building blocks combine, and create useful materials with specific characteristics by manipulating the molecular structure of the monomers/polymers used, the composition of the monomer/polymer combinations, and applying chemical and processing techniques that can to a large extent, affect the properties of the final product. Polymer chemists are unique within the chemistry community because their understanding of the relationship between structure and property spans from the molecular scale to the macroscopic scale.
Q2) Enlist the properties of polymers.
A2)
- Polymers can be very resistant to chemicals.
- Polymers can be both thermal and electrical insulators.
- Generally, polymers are light in weight with varying degrees of strength.
- Polymers can be processed in various ways to produce thin fibers or intricate parts.
- A polymer is a molecule formed by the joining of thousands of smaller molecular units together by chemical bonds. A chemical process that leads to the formation of a polymer is known as polymerization.
Q3)Classify polymers.
A3)
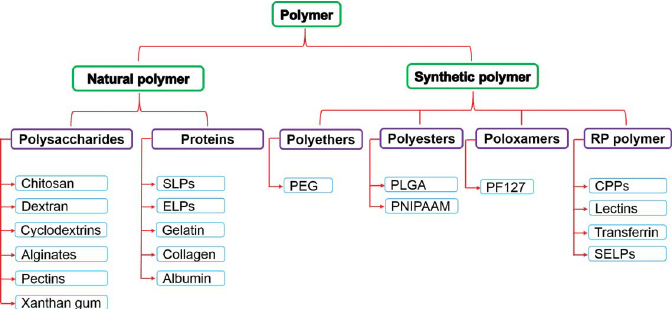
|
Q4) Explain addition polymerization.
A4) The addition polymerization is the process in which the linking together of monomer molecules by a chain reaction is observed. Polymer synthesized by addition polymerization has the same empirical formula as that of monomer. No molecule is evolved during polymerization and the polymer is an exact multiple of the original monomeric molecule.
Q5)Explain the free radical mechanism of addition polymerization.
A5)
The formation of polymer from the successive addition of free-radical building blocks through the polymerization is called free radical polymerization. In order to obtain a wide variety of different polymers and material composites, free radical polymerization plays a major role in it. The relatively non-specific nature of free-radical chemical interaction makes it one of the most versatile forms of polymerization which allow a facile reaction of polymeric free radical chain ends and other chemical or substrates. The monomers from which addition polymers are made are alkenes. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates, and this is the mechanism followed by most polymerization. The monomers are initiated by traces of oxygen, the pure compounds are stabilized by radical inhibitors.  Radical Initiators 
Chain Initiation 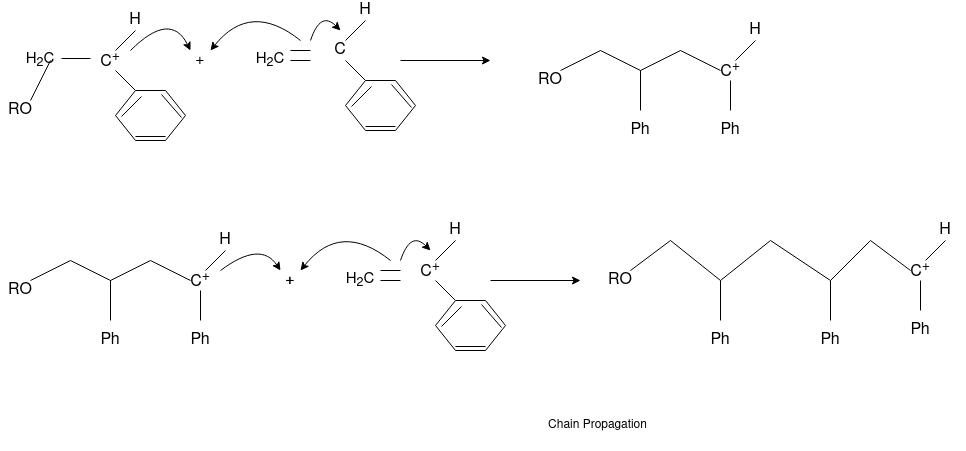
Chain Propagation
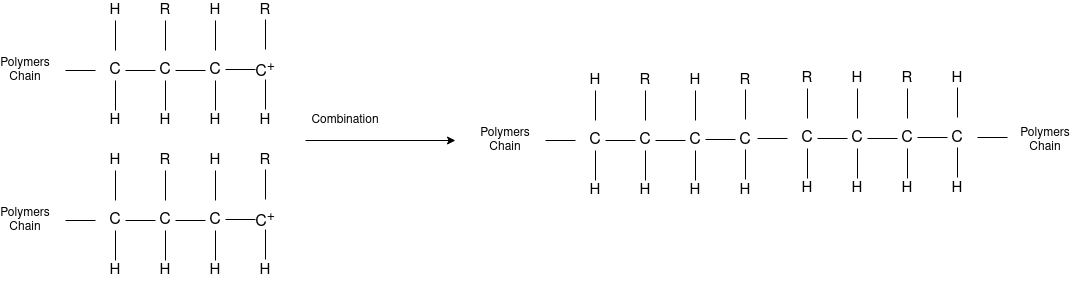 Chain Termination 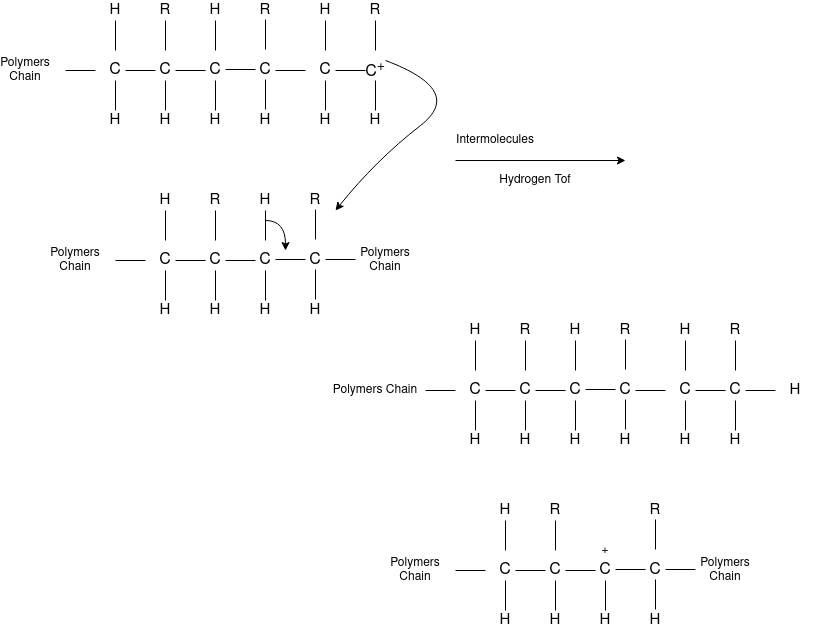
Chain Transfer Reaction |
Q6)What are plastics?
A6) Synthetic or semi-synthetic polymers are plastics. Plastics used for industrial work come from petrochemicals. Plastic refers to its ability to deform without breaking. The polymer used in making plastics is usually a combination of additives, colorants, plasticizers, stabilizers, fillers, and reinforcements. These additives affect the chemical composition, properties, and mechanical properties of plastics and affect their cost.
Q7) What are thermoplastic polymers?
A7) Thermoplastic polymer: A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating, flowing due to crystal melting or by virtue of crossing the glass transition temperature (Tg). Upon processing, usually via injection-molding or blow-molding-like processes, thermoplastics take the shape of the mould within which they are poured as melt, and cool to solidify into the desired shape. The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape. This allows for additional processing of the same material, even after being prepared as a solid. Processes such as extrusion, thermoforming, and injection molding rely on such resin behavior. Some common thermoplastic materials include polyethylene (PE), polycarbonate (PC), and polyvinyl chloride (PVC).
Q8) Enlist the properties of thermosetting and thermoplastic polymers.
A8)
Property | Thermoplastics Polymer | Thermosetting Polymer |
Molecular Structure | Linear polymer: weak molecular bonds in a straight-chain formation | Network polymers: high level of crosslinking with strong chemical molecular bonds |
Melting point | Melting point lower than the degradation temperature | Melting point higher than the degradation temperature |
Mechanical | Flexible and elastic. High resistance to impact (10x more than thermosets). Strength comes from crystallinity | Inelastic and brittle. Strong and rigid. Strength comes from crosslinking. |
Polymerization | Addition polymerisation: repolymerised during manufacture (before processing) | Polycondensation polymerisation: polymerised during processing |
Microstructure | Comprised of hard crystalline and elastic amorphous regions in its solid-state | Comprised of thermosetting resin and reinforcing fibre in its solid-state |
Size | Size is expressed by molecular weight | Size is expressed by crosslink density |
Solubility | Can dissolve in organic solvents | Do not dissolve in organic solvents |
Service temperature | Lower continuous use temperature (CUT) than thermosets | Higher CUT than thermoplastics |
Q9) Explain vulcanization of rubber.
A9) Vulcanization is a chemical process that converts natural rubber and other polydiene elastomers into cross-linked polymers. The most common vulcanization agent is sulfur. It forms bridges between individual polymer molecules when heated with rubber. Often a catalyst and initiator are added to accelerate the vulcanization process. The cross-linked elastomers have many improved mechanical properties. In fact, unvulcanized rubber has poor mechanical properties and is not very durable.
- Mixing of crude rubber with about 5-30% of sulfur (cross-linking agent) and other additives such as:
- Activator (commonly zinc oxide or stearic acid),
- Accelerator (guanidines, thiazoles, dithiocarbamates, xanthates, thiurams,
- Coagulants (acetic acid, calcium chloride),
- Anti-oxidants (amines, phenolics, phosphites),
- Color pigments,
- Surfactants,
- Softeners,
- Ant-foaming agents,
- Anti-tack agents (Rosin derivates, coumarone-indene resins, aliphatic petroleum resins, alkyl-modified phenol-formaldehyde resins).
Slow cross-linking starts at this stage. It is necessary to avoid active vulcanization during mixing, which may cause cracks formation at the molding stage.
- Molding (shaping) the rubber mixture. The rubber must be shaped prior to the heating stage since cross-linking makes shaping impossible.
- Heating the mixture to 250-400ºF (120-200ºC). The increased temperature speeds up the vulcanization process resulting in fast and complete cross-linking. C-S bonds replace C-H bonds linking chain poly-isoprene molecules. Each link is formed by one to seven sulfur atoms.
Q10) Enlist application of vulcanized rubber.
A10)
Applications:
(i) The major application of natural rubber is in the manufacture of tyres.
(ii) In heavy-duty tyres, the major portion of the rubber used is natural rubber.
(iii) The tank linings in chemical plants where corrosive chemicals are stored are prepared from rubber.
(iv) To reduce machine vibrations, rubber is used for sandwiching between two metal surfaces.
(v) Foam rubber is used for making cushions’, matrices, padding, etc. toys and sports items are manufactured from natural rubber.
Q11)Explain the preparation of Buna-S and its applications.
A11)
Preparation of Buna-S: Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrene. Buna-S is the replacement of natural rubber while styrene, 2 monomers, and butadiene play a major role in its derivation whereas these 2 monomers are polymerized by two different processes i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene. It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called cold rubber. The obtained rubber is called Styrene-Butadiene Rubber (SBR). 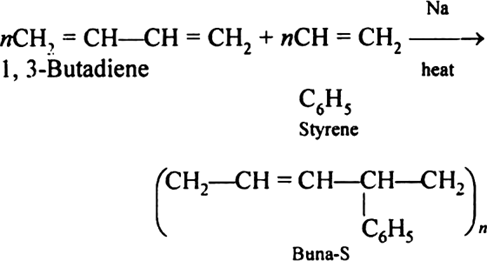 Applications: (i) Buna-S is used as the natural rubber i.e., they are widely used in pneumatic tyres. (ii) SBR is extensively used in coated papers. (iii) They are highly used in building materials as a binding material. (iv) SBR is also used as a binder in lithium-ion battery electrodes. |
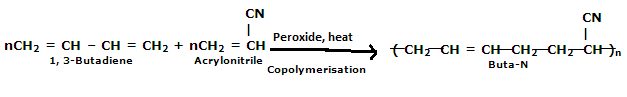
Q12)Explain the preparation of Buna-N and its applications.
A12)
Preparation of Buna-N:
Buna-N is commonly used as elastomers. They are unsaturated synthetic copolymers. They are obtained by the copolymerisation of 1,3-butadiene and acrylonitrile in the presence of a peroxide catalyst. Nitrile rubber, NBR, or Perbunan are other terms used for the Buna-N. They are synthetic rubber copolymer of acrylonitrile (ACN) and butadiene. Elastomers are natural or synthetic material that does not break when stretched and when released it returns to its original length. The most important monomer used in preparing Buna-N is Acrylonitrile rubber which gives hardness, tensile strength, fuel, and oil resistance. It usually contains 34% ACN. Grinding wheels can be made with nitrile rubber. When they are used for grinding smokes or fumes are emitted, which are known as acrid. The objectionable odor can be prevented if certain classes of diketones, which contain a conjugated system of two double bonds or unsaturated groups are added. Dibenzoylethylene, chloranil, and anthraquinone help to prevent odor formation.
|
Buna-N is resistant to oil, fuel, and other chemicals which means more Buna-N higher the resistance to oil but the flexibility of the material is less. They are resistant to aliphatic hydrocarbons. They are non-resistant to solvents but they may be attacked by ozone, ketones, esters, and aldehydes.
Applications:
(i) They are used in disposable non-latex rubbers, belts, gaskets.
(ii) They are used in the preparation of adhesive and as a pigment binder.