Unit-2
Abrasives and Adhesives
Q1) What are abrasives?
A1)Abrasive is a sharp and hard material that is used to wear away the surface of softer, less resistant materials. Included within the term are both natural and synthetic substances, ranging from the relatively soft particles used in household cleansers and jeweler’s polish to the hardest known material, the diamond.
Abrasives are used in the form of grinding wheels, sandpapers, honing stones, polishes, cutoff wheels, tumbling and vibratory mass-finishing media, sandblasting, pulp stones, ball mills, and still other tools and products. Only through the use of abrasives is the industry able to produce the highly precise components and ultra-smooth surfaces required in the manufacture of automobiles, airplanes and space vehicles, mechanical and electrical appliances, and machine tools.
Q2)Enlist the properties and uses of corundum.
A2)
Corundum (Al2O3) [Alundum]
Properties :
- Crystalline
- Very Hard
- Moh’s scale-9
- Brown to grey in color
Uses :
- In grinding wheels
- To grind glass/lens/metals,
- Ruby lasers
Q3) Enlist the properties and uses of Diamond.
A3)
- Diamond exists in three major forms
- Diamond (gem-grade)
- Borts – These are diamonds that are off colour or faulty
- Carbonado – These are black diamonds mined from Brazil. They have good hardness, but due to lackluster, do not find application as jewelry.
- They are commonly used as abrasives
Properties
- Crystalline
- Chemically inactive
- Moh’s scale-10
Uses
- In bits of drilling points
- Sawteeth for cutting rocks
- In grinding wheels
- In engraving tools
Q4) Explain Silicon Carbides.
A4)
Preparation: Raw materials are
All raw materials are sized, dried, and mixed along with old charge and fed into the Acheson furnace with a little amount of NaCl (flux) 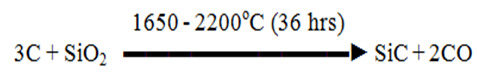 Properties Melting Point is 2700oC Mohs scale hardness is 9.3 Chemically Inert High Thermal Stability Brittle hence strength is less USES Cutting tools Grinding of cast iron, brass, bronze, porcelain marble Polishing leather, lenses (Abrasive paper and Cloth) Refractory in furnace |
Q5)What is cement? Classify it.
A5)Concrete is the most widely used non-metallic material in the construction of buildings, dams, bridges, etc. Cement act as a binder substance that is used to harden and help in binding the material together. It is used for binding material like bricks, stones, tile and also used for flooring and plastering purposes. Cement is used for construction work by mixing up sand and crushed stones. It is a mixture of sand and water which is used in binding bricks together and for plastering purpose. Based on chemical composition cement can be classified into 4 different parts:
(i) Natural Cement
(ii) Puzzolana Cement
(iii) Slag Cement
Q6)Classify Abrasives.
A6)
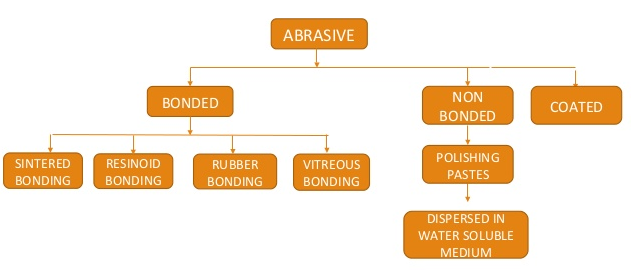
|
Q7)What are wet process manufacturing of cement?
A7)On using the calcareous as raw material then chalk must be crushed into minute particles and hence dispersed in water in a wash mill. Wash mill used to break the solid materials into lumps/slurry. In similar wash mill clay is also broken up and mixed with water. Then this slurry is stored in the silo called slurry silo where it is consistently stirred. The composition of raw material is checked again and if required corrected by adding clay or chalk materials as per requirement.
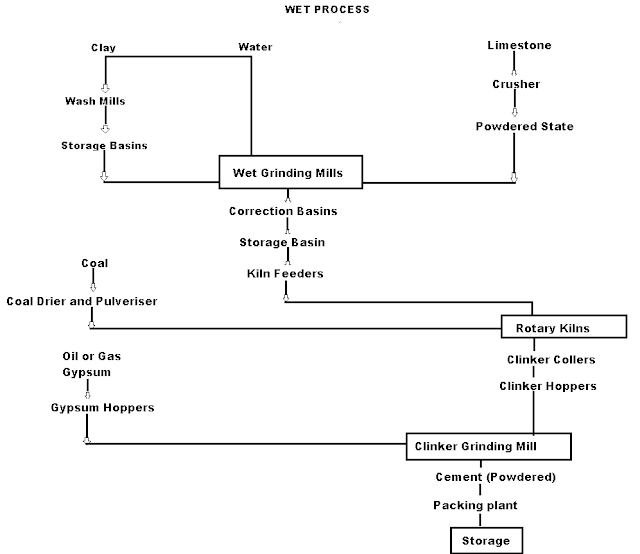 |
Cement Manufacturing Process Flow Chart
(i) Drying Zones: The raw material in slurry form is directly fed into the kiln which has more amount of water. It is the upper portion of the kiln. In this zone, water is evaporated at temperatures 100-400°C.
(ii) Formations of modules: As the slurry gradually descends in the kiln, the carbon dioxide from the slurry evaporates and small lumps formed which may be called modules.
(iii) Burning Zone:- The modules enter this zone where temperatures are kept about 1400-1500° C. The modules are converted into dark greenish balls and the product obtained in the kiln, known as clinker, is of varying size 5 to 20 mm. The clinkers are very hot when coming out of this zone.
(iv) Cooling of Clinkers:- It is used to cool down the clinkers up to about 90°C.
Grinding: The cooled clinkers are finally ground in ball mills or tube mills.
Also, the gypsum is added during grinding about 2-4%. The gypsum acts as a retarder and so allows the cement to mix with sand or aggregate and to be placed in position. i.e. it increases the initial setting time of cement.
Storage and Packing: As cement comes out from grinding mills, it is collected in a hopper and taken in a bucket elevator for storage in silos.
The cement from silos is packed by machines in bags. Each bag of cement contains 50 kg or 0.035 m3 of cement.
Q8) What are dry process manufacturing of cement?
A8)Dry Process manufacturing: This type of process for manufacturing cement is available in the places where hard crystalline limestone and shales are available. The advantage of using this type of process is that fuel consumption is low.
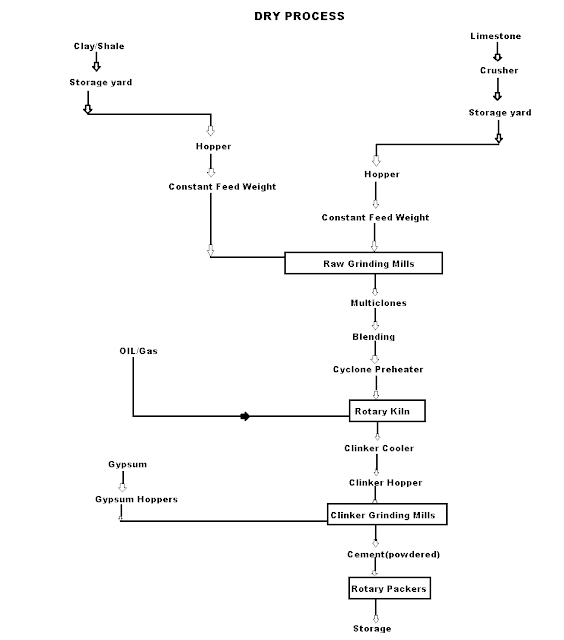
|
Mixing of Raw Materials: The used raw material undergo the following process:
- Crushing: The raw materials are broken in crushers to small fragments.
- Drying: The crushed materials are dried by heating at a sufficiently high temperature. It may be done in drying kilns.
- Reduction of size: The drying materials are then ground by using ball mills and tube mills to reduce the size of materials to find powder.
- Mixing in correct proportion: The finely dried materials are mixed in exact proportions. The mixing may be done either mechanically or by pneumatic methods.
Burning and Grinding: In this process, the raw materials are mixed, fined, and then fed into the kiln whereas, in the wet process, the raw materials are crushed separately and then directly mixed in correct proportion in the presence of water to make a fine thin paste known as Slurry.
Q9) Enlist the composition of Portland cement.
A9)
Composition of Portland Cement: - Hydraulic cement produced by pulverizing clinkers that consist of calcium silicate, usually containing one or more of the forms of calcium sulfate as an inter ground station. A good sample of Portland cement consists of: (i) Silica(SiO2): - 20-24% (ii) Calcium Oxide: -60-70% (iii) Alumina (Al2O3): -5-7% (iv) Magnesia(MgO): -2-3% (v) Ferric Oxide (Fe2O3): -1-2.5% (vi) Sulphur trioxide (SO3): -1-1.5% (vii) Sulphur Oxide: -1% (viii) Potassium Oxide (K2O): -1% |
Q10)Classify Adhesives.
A10)
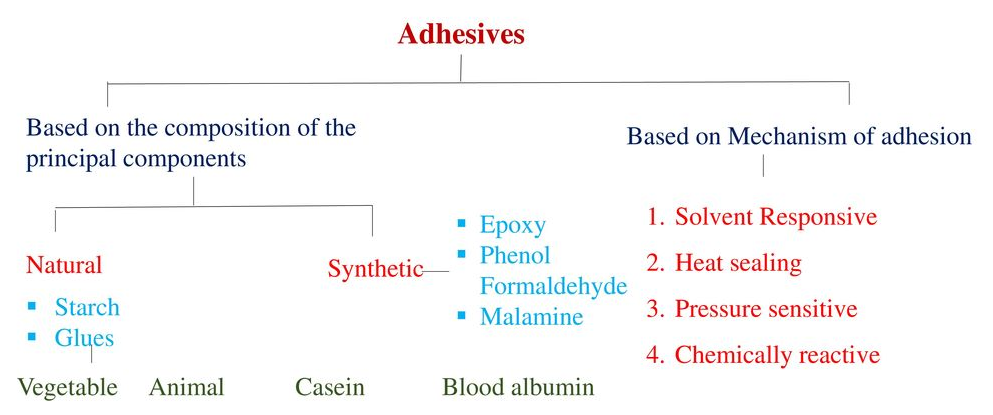
|
Q11)What are the physical factors that affect adhesive action?
A11)
1) Surface tension
For better bond strength interfacial tension between the adhesives molecules and surface material of adherents should be less.
2) Shear compressive and tensile strength of the adhesive
The shear compressive and tensile strength of adhesive indirectly contributes to the load-bearing capacity of the composite material. Thus for high strength of composites material adhesive material, in turn, should have high strength.
3) Porosity and smoothness of contacting surfaces
Porosity is a physical factor that indicates bond strength between surface molecules. If the porosity is high then the bond is low and vice-versa. While high smoothness is a sign of good strength. High smoothness ensures the absence of impurities on any surface. Thus for any contacting surface porosity should be less and smoothness should be as high as possible.