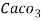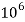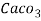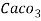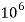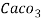Unit 3
Water Technology
Q1)What is hardness?
A1)
Hardness can be defined as the soap consuming capacity of the water sample. Soaps are sodium salts of fatty acids like oleic acid, palmitic acid, and stearic acid. They dissolve readily .in water to form lather due to which it has cleansing property.

 (calcium stearate)
|
Q2)Explain temporary hardness.
A2)
Temporary hardness ( carbonate):-

Mg 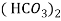    (Bicarbonates) |
Q3)Explain the term permanent hardness.
A3)
Permanent hardness: -
- The term permanent hardness or non-carbonate is the term applied to the hardness caused by dissolved chlorides, nitrates, and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- The Sum of temporary and permanent hardness is referred to as total hardness.
Q4)Explain the estimation of hardness by the complexometric method.
A4)
Complex metric titration is a form of volumetric analysis in which the formation of a colored complex is used to indicate the endpoint of a titration. Complex metric titrations are particularly useful for the determination of a mixture of different metal ions in solution. An indicator capable of producing an unambiguous color change is used to detect the end-point of the titration. Complexometric titration is those reactions where a simple ion is transformed into a complex ion and the equivalence point is determined by using metal indicators or electrometrically.
- The reaction reaches equilibrium rapidly after each portion of titration is added.
- Interfering situations do not arise.
- A complexometric indicator capable of locating equivalence point with fair accuracy is available.
Titration with EDTA
EDTA(Ethylene Diamine Tetra Acetic Acid), has four carboxyl groups and two amine groups that can act as electron-pair donors. The ability of EDTA to potentially donate its six lone pairs of electrons for the formation of coordinate covalent bonds to metal cations makes EDTA a hexadentate ligand. However, in practice, EDTA is usually only partially ionized and thus forms fewer than six-coordinate covalent bonds with metal cations.
Disodium EDTA is commonly used to standardize aqueous solutions of transition metal cations. Disodium EDTA (often written as Na2H2Y) only forms four coordinate covalent bonds to metal cations at pH values ≤ 12. In this pH range, the amine groups remain protonated and thus unable to donate electrons to the formation of coordinate covalent bonds. Note that the shorthand form Na4−xHxY can be used to represent any species of EDTA, with x designating the number of acidic protons bonded to the EDTA molecule.
EDTA forms an octahedral complex with most 2+ metal cations, M2+, in an aqueous solution. The main reason that EDTA is used so extensively in the standardization of metal cation solutions is that the formation constant for most metal cation-EDTA complexes is very high, meaning that the equilibrium for the reaction:
M2+ + H4Y → MH2Y + 2H+
Lies far to the right. Carrying out the reaction in a basic buffer solution removes H+ as it is formed, which also favors the formation of the EDTA-metal cation complex reaction product. For most purposes, it can be considered that the formation of the metal cation-EDTA complex goes to completion, and this is chiefly why EDTA is used in titrations and standardizations of this type.
Q5)Enlist the different units of hardness.
A5)
Thus, 1 mg/l = 1 mg of  But 1 L OF Water weighs. = 1 kg = 1000 g = 1000*1000 mg =  Therefore, 1mg/l = 1 mg of 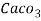  = 1 part of 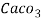  = 1 part of 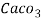 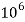 =1 ppm
3. Clarke’s degree is the number of grains (1/7000lb) of   Thus, 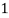 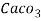 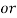 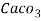
4. Degree French (°fr) is the part of 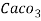 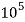 Thus, 1°fr = 1 part of 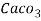 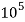
5. Milli equivalent per liter (meq / L )is the number of milliequivalents of hardness present per liter. Thus, 1meq/L = 1 meq of 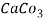 = 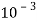 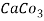 = 50 mg of  = 50 mg / l of 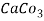 |
Q6)What is the relationship between the different unit of hardness?
A6)
|
Q7) Enlist the disadvantage of hard water.
A7) Disadvantages of using hard water are-
1. Hard water is unfit for washing as it is difficult to form lather with soap.
2. Scum may form in a reaction with soap, wasting the soap.
3. Furring of tea kettles will take place due to the formation of carbonates of calcium and magnesium.
4. Hard blocks hot water pipes. This is due to the formation of layers of carbonates of calcium and magnesium.
Q8)Difference between scale and sludge.
A8)
Sludge | Scale |
Sludge is a loose, soft, and slimy precipitate | Scale forms hard deposits |
Sludge from non-adherent deposits and can be easily removed | Scale sticks firmly to the inner surface of the boiler and is very difficult to remove. |
Sludge is formed by a substance like CaCl2, MgCl2, MgSO4, etc. | The scale is formed by a substance like CaSO4, Mg(OH)2, etc. |
Sludge is formed at comparatively colder portions of the boiler. | The scale is formed at heated portions of the boiler |
They decrease the efficiency of the boiler but are less dangerous | They decrease the efficiency of the boiler and chances of the explosion are also there. |
They can be removed by blowdown operation | They can’t be removed by blowdown operation. |
Q9)Enlist the disadvantages of priming and foaming.
A9)Priming and Foaming usually occur together. They are objectionable because:-
1. The dissolved salts in boiler water are carried by the wet steam to the superheater and turbine blades where they get deposited and reduce the efficiency.
2. The dissolved salts enter the parts of other machinery where steam is used thereby decreasing the life of machinery.
3. The actual height of the water column cannot be judged thereby making the maintenance of boiler pressure becomes difficult.
Q10)Explain the Zeolite process.
A10)
The zeolite process is a process of softening hard water through an ion-exchange technique using a chemical compound zeolite. It possesses a chemical compound that has hydrated sodium alumina silicate. Thus, the name of the process is called a zeolite process. Zeolite compound can exchange its sodium cations reversibly with calcium and magnesium ions in the water softening process.
There are two types of zeolite used in this process they include natural and synthetic zeolite. The natural form is found to be porous and the synthetic form is a non-porous zeolite. However synthetic form possesses a high exchange capacity per unit weight than the natural form.
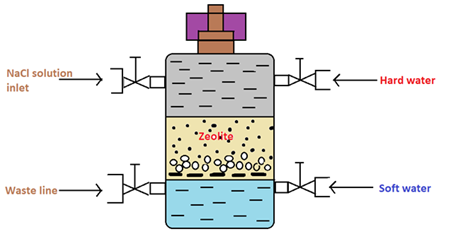
|
Figure 01: Cylinder Containing the Zeolite Bed
Process
In the water softening process, hard water is passed through a bed of zeolite (inside a cylinder) at a specified rate. Then the cations that cause the water-hardening will remain on the zeolite bed because these cations exchange with the sodium cations of zeolite. Therefore, the water coming out of this cylinder contains sodium cations rather than calcium and magnesium cations.
After some time, the zeolite bed gets exhausted, the water flow is stopped, and treat the bed is treated with concentrated brine solution (10%) to regenerate the zeolite. The bed is treated with a brine solution, as it washes away all the calcium and magnesium ions, by exchanging them with sodium ions in a brine solution. Hence, this treatment regenerates the zeolite.
Q11) Enlist the application of pH meter.
A11)
- The measurement of pH reflects the effective concentration and activity of H+ and other ions present in solutions
- For chemical reactors and scrubbers, they provide indications of the solution used being acidic or basic qualitatively
- These meters find major application to correct the hyper chloric concentration for an oxidation-reduction potential measurement.
- Water treatment plants, micro-electronics laboratories, and pharmaceutical laboratories are in constant need of pH level monitoring and control for their very accurate and precise applications.
Unit 3
Water Technology
Q1)What is hardness?
A1)
Hardness can be defined as the soap consuming capacity of the water sample. Soaps are sodium salts of fatty acids like oleic acid, palmitic acid, and stearic acid. They dissolve readily .in water to form lather due to which it has cleansing property.

 (calcium stearate)
|
Q2)Explain temporary hardness.
A2)
Temporary hardness ( carbonate):-

Mg    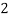 (Bicarbonates) |
Q3)Explain the term permanent hardness.
A3)
Permanent hardness: -
- The term permanent hardness or non-carbonate is the term applied to the hardness caused by dissolved chlorides, nitrates, and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- The Sum of temporary and permanent hardness is referred to as total hardness.
Q4)Explain the estimation of hardness by the complexometric method.
A4)
Complex metric titration is a form of volumetric analysis in which the formation of a colored complex is used to indicate the endpoint of a titration. Complex metric titrations are particularly useful for the determination of a mixture of different metal ions in solution. An indicator capable of producing an unambiguous color change is used to detect the end-point of the titration. Complexometric titration is those reactions where a simple ion is transformed into a complex ion and the equivalence point is determined by using metal indicators or electrometrically.
- The reaction reaches equilibrium rapidly after each portion of titration is added.
- Interfering situations do not arise.
- A complexometric indicator capable of locating equivalence point with fair accuracy is available.
Titration with EDTA
EDTA(Ethylene Diamine Tetra Acetic Acid), has four carboxyl groups and two amine groups that can act as electron-pair donors. The ability of EDTA to potentially donate its six lone pairs of electrons for the formation of coordinate covalent bonds to metal cations makes EDTA a hexadentate ligand. However, in practice, EDTA is usually only partially ionized and thus forms fewer than six-coordinate covalent bonds with metal cations.
Disodium EDTA is commonly used to standardize aqueous solutions of transition metal cations. Disodium EDTA (often written as Na2H2Y) only forms four coordinate covalent bonds to metal cations at pH values ≤ 12. In this pH range, the amine groups remain protonated and thus unable to donate electrons to the formation of coordinate covalent bonds. Note that the shorthand form Na4−xHxY can be used to represent any species of EDTA, with x designating the number of acidic protons bonded to the EDTA molecule.
EDTA forms an octahedral complex with most 2+ metal cations, M2+, in an aqueous solution. The main reason that EDTA is used so extensively in the standardization of metal cation solutions is that the formation constant for most metal cation-EDTA complexes is very high, meaning that the equilibrium for the reaction:
M2+ + H4Y → MH2Y + 2H+
Lies far to the right. Carrying out the reaction in a basic buffer solution removes H+ as it is formed, which also favors the formation of the EDTA-metal cation complex reaction product. For most purposes, it can be considered that the formation of the metal cation-EDTA complex goes to completion, and this is chiefly why EDTA is used in titrations and standardizations of this type.
Q5)Enlist the different units of hardness.
A5)
Thus, 1 mg/l = 1 mg of 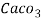 But 1 L OF Water weighs. = 1 kg = 1000 g = 1000*1000 mg = 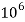 Therefore, 1mg/l = 1 mg of 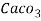 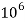 = 1 part of 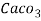  = 1 part of  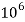 =1 ppm
3. Clarke’s degree is the number of grains (1/7000lb) of  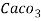 Thus, 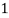 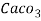 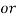 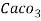
4. Degree French (°fr) is the part of 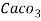  Thus, 1°fr = 1 part of 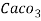 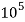
5. Milli equivalent per liter (meq / L )is the number of milliequivalents of hardness present per liter. Thus, 1meq/L = 1 meq of 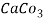 = 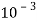 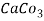 = 50 mg of 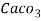 = 50 mg / l of 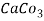 |
Q6)What is the relationship between the different unit of hardness?
A6)
|
Q7) Enlist the disadvantage of hard water.
A7) Disadvantages of using hard water are-
1. Hard water is unfit for washing as it is difficult to form lather with soap.
2. Scum may form in a reaction with soap, wasting the soap.
3. Furring of tea kettles will take place due to the formation of carbonates of calcium and magnesium.
4. Hard blocks hot water pipes. This is due to the formation of layers of carbonates of calcium and magnesium.
Q8)Difference between scale and sludge.
A8)
Sludge | Scale |
Sludge is a loose, soft, and slimy precipitate | Scale forms hard deposits |
Sludge from non-adherent deposits and can be easily removed | Scale sticks firmly to the inner surface of the boiler and is very difficult to remove. |
Sludge is formed by a substance like CaCl2, MgCl2, MgSO4, etc. | The scale is formed by a substance like CaSO4, Mg(OH)2, etc. |
Sludge is formed at comparatively colder portions of the boiler. | The scale is formed at heated portions of the boiler |
They decrease the efficiency of the boiler but are less dangerous | They decrease the efficiency of the boiler and chances of the explosion are also there. |
They can be removed by blowdown operation | They can’t be removed by blowdown operation. |
Q9)Enlist the disadvantages of priming and foaming.
A9)Priming and Foaming usually occur together. They are objectionable because:-
1. The dissolved salts in boiler water are carried by the wet steam to the superheater and turbine blades where they get deposited and reduce the efficiency.
2. The dissolved salts enter the parts of other machinery where steam is used thereby decreasing the life of machinery.
3. The actual height of the water column cannot be judged thereby making the maintenance of boiler pressure becomes difficult.
Q10)Explain the Zeolite process.
A10)
The zeolite process is a process of softening hard water through an ion-exchange technique using a chemical compound zeolite. It possesses a chemical compound that has hydrated sodium alumina silicate. Thus, the name of the process is called a zeolite process. Zeolite compound can exchange its sodium cations reversibly with calcium and magnesium ions in the water softening process.
There are two types of zeolite used in this process they include natural and synthetic zeolite. The natural form is found to be porous and the synthetic form is a non-porous zeolite. However synthetic form possesses a high exchange capacity per unit weight than the natural form.
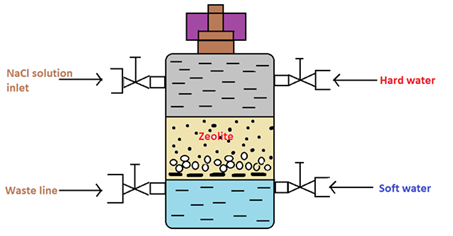
|
Figure 01: Cylinder Containing the Zeolite Bed
Process
In the water softening process, hard water is passed through a bed of zeolite (inside a cylinder) at a specified rate. Then the cations that cause the water-hardening will remain on the zeolite bed because these cations exchange with the sodium cations of zeolite. Therefore, the water coming out of this cylinder contains sodium cations rather than calcium and magnesium cations.
After some time, the zeolite bed gets exhausted, the water flow is stopped, and treat the bed is treated with concentrated brine solution (10%) to regenerate the zeolite. The bed is treated with a brine solution, as it washes away all the calcium and magnesium ions, by exchanging them with sodium ions in a brine solution. Hence, this treatment regenerates the zeolite.
Q11) Enlist the application of pH meter.
A11)
- The measurement of pH reflects the effective concentration and activity of H+ and other ions present in solutions
- For chemical reactors and scrubbers, they provide indications of the solution used being acidic or basic qualitatively
- These meters find major application to correct the hyper chloric concentration for an oxidation-reduction potential measurement.
- Water treatment plants, micro-electronics laboratories, and pharmaceutical laboratories are in constant need of pH level monitoring and control for their very accurate and precise applications.