Unit 4
Corrosion Science and Lubricants
Q1)Define corrosion.
A1)Corrosion is defined as the phenomenon in which metal get a coated covering over its whole body due to the chemical reaction from its surrounding that results in the conversion of metal into the oxide, salt, or any other compound. In common language, this process is called the rusting of iron. The theories behind the corrosion of the metal are:
1-Direct Corrosion
2-Electro-Chemical Corrosion
3-High-Temperature Oxidation
Q2)What is chemical corrosion?
A2)The reaction of metal with water vapour or gas at high temperature causes the metal to corrode chemically. This is the redox process in which the electron of the metal is passed directly to the substance in the environment. The metal corrodes generally in the metal which is in higher contact with water.
 3Fe + 4H2O Fe3O4 + 4H2
3Fe + 4H2O Fe3O4 + 4H2
 3Fe + 2O2 Fe3O4
3Fe + 2O2 Fe3O4
Q3)What is electrochemical corrosion?
A3)Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution, electrons are released by the metal and that is gained by the elements in the corroding solution. The release of an electron from metal is called oxidation while vice-versa that is the gain of an electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of the metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
When wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
Q4)Enlist the factors that affect corrosion.
A4) (i)Nature of the metal
Position of metal in galvanic series.
If the position is higher in galvanic series then it corrodes faster
While for 2 metals the difference between them shows the corrosion ratio.
(ii)Potential Difference
If the difference at the electrode potential between two metals is high then the rate of corrosion would be also high while vice versa for the lesser difference.
(iii)Purity of metal
Corrosion never took place in pure metals. While if the metal itself has an impurity then galvanic cells are set up easily which intend increases the rate of corrosion.
(iv)Relative areas of cathode and anode parts
The rate of corrosion directly depends on the area of the cathode and inversely depends on the area of the anode. If the area of cathode is larger then there is more demand for electrons while in the smaller anode area the corrosion took place very fast.
(v)Nature of corrosion Product
The metal oxide film is formed on the surface of the metal by corrosion due to oxygen. The formed film would be stable, unstable, volatile.
(vi)Temperature
At high temperatures, the rate of corrosion increases because there is a consistent increase in the ionization and mobility difference rate while in some cases rate of corrosion decreases at high temperatures as the solubility of O2 gas increases.
(vii)Presence of moisture
The rate of corrosion decreases in dry while increases in presence of moisture. Moisture act as the solvent for setting up electrochemical corrosion.
(viii)Effect of pH
The rate of corrosion is high at acidic pH due to the evolution of H2 gas at the cathode.
(ix)Concentration of electrolytes
This is also called the Oxygen concentration cell. The rate of corrosion would directly depend on the supply of oxygen in the air.
(x)Over Voltage
The difference between the actual value and theoretical value of the decomposition potential of the electrode.
Q5)Enlist the corrosion protection method.
A5)
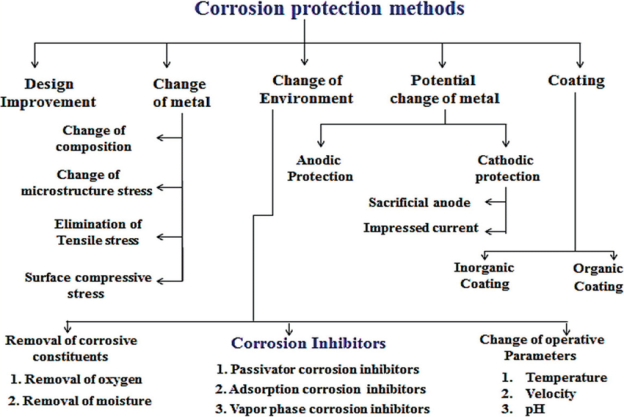 |
Q6)Explain Phosphating.
A6)The conversion of surface metal atoms into their phosphates by chemical or electrochemical reactions is called phosphating.
The phosphating bath contains three essential components:
1) free phosphoric acids
2) a metal phosphoric such as Fe, Mn phosphate
3) An accelerator such as H2O2, nitrites, nitrates
4) Temperature – 35oC
5) pH – 1.8 – 3.2
Phosphating not only improves the corrosion resistance but also imparts good paint adhesion quality to the surface.
Q7)What are galvanizing methods? Enlist its applications.
A7)Galvanizing:
Method:
- Coating of Zn on the steel or iron is called galvanizing.
- Steel article is cleaned well with dil H2SO4 then with water and dry it.
- Then the steel article is dipped in molten Zn bath and it is maintained at the temp 425 – 450 c.
- The molten Zn bath is covered with flux.
- Then the article is allowed to pass through to mate uniform coating layer and to remove excesses of Zn.
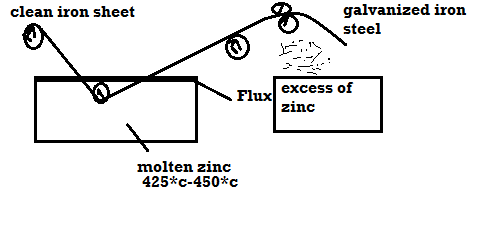 |
Applications: -
- Various galvanized iron (G.I) articles are used.
- It is useful for the protection of iron.
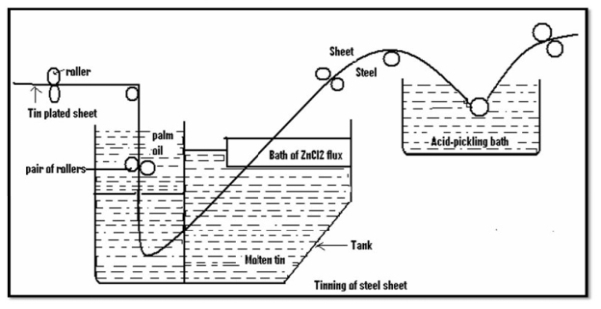
Q8) What are tinning?
A8)
Method: -
- Coating of tin on a base metal i.e steel iron is called tinning.
- The steel article is cleaned well and then passes through a molten tin bath which is maintained at temp 240c.
- Then, the article is allowed to pass through a palm oil bath which protects the hot tin-coated surface against corrosion.
- Then the article is allowed to pass through to make a uniform coating layer to remove the excess of tin.
|
Q9) Explain Sacrificial Anodic Protection.
A9)The metal surface can be protected from corrosion by connecting its wire to a more anodic metal. The sacrifice of this more anodic metal to save the metal from corrosion is called the Sacrificial Anode. The most common metal used for this purpose is Mg, Zn, Al, etc.
Applications:
(i) The underground cable and pipeline protection from soil erosion.
(ii) Ships and boat protection from marine corrosion.
(iii) Prevention of rusty water by inserting Mg sheets or rods into domestic water boiler or tanks.
Q10)What are semi-solid lubricants?
A10)Lubricating grease is a semi-solid, consisting of a soap dispersed throughout liquid lubricating oil. The liquid lubricant may be petroleum oil or even synthetic oil and it may contain any of the additives for specific requirements. Greases are prepared by saponification of fat with an alkali, followed by adding hot lubricating oil while under agitation. The total amount of mineral oil added determines the consistency of the finished grease. The structure of lubricating greases is that of a gel. Soaps are gelling agents, which give an interconnected structure containing the added oil. At high temperatures, the soap dissolves in the oil, whereupon the interconnected structures cease to exist and the grease liquefies. The consistency of greases may vary from a heavy viscous liquid to a stiff solid mass. To improve the heat-resistance of grease, inorganic solid thickening agents (like finely divided clay, bentonite, colloidal silica, carbon black, etc.) are added.
Greases have higher shear or frictional resistance than oils and, therefore, can support much heavier loads at lower speeds. They also do not require as much attention, unlike the lubricating liquids. But greases tend to separate into oils and soaps.
Grease is used:
(i) in situations where oil cannot remain in place, due to high load, low speed, intermittent operation, sudden jerks, etc. e.g. Rail axle boxes,
(ii) in bearing and gears that work at high temperatures
(iii) in situations where bearing needs to be sealed against entry of dust, dirt, grit, or moisture because greases are less liable to contamination by these
(iv) in situations where dripping or spurting of oil is undesirable because unlike oils, greases if users do not splash or drip over articles being prepared by the machine. For example, in machines preparing the paper, textiles, edible articles, etc.
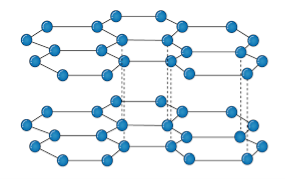
Q11)Explain graphites. Its structure and applications.
A11)It is an allotropic form of the carbon atom (Allotropy form- When any element is formed in more than one form and each form, they possess different property). In graphite, carbon is arranged in hexagonal ring form and each form is arranged upside down to form the layer and is attached by weak Vander-wall’s forces. Graphite is the most stable form of carbon. It is a good conductor of heat and electricity. The two major known forms of graphite are alpha (Hexagonal) and Beta (Rhombohederal).
Structure:
The carbon atom is sp2 hybridized that means there is 4 valence electron in their outermost shell as in the diamond the all 4 valence electron is covalently bonded while in graphite the 3 electron makes a covalent bond while the remaining 1 electron is free. The interconnection of these carbon atoms forms the hexagonal structure. The C-C bond length is 1.42 Angstrom. This hexagonal layer is attached toward each other by weak Vander Wall forces at a distance of 3.35 Angstrom. This is the reason why graphite is soft.
 |
Properties:
Conducting Property: As the carbon of Graphite is sp2 hybridized that means the p orbital remains vacant of the carbon atom which tends to the overlapping of the vacant P-orbital. This overlapping of the vacant orbital is responsible for the movement of electrons in parallel on their vacant orbital that is why Graphite shows the conducting property.
Lubricating Property: There is the presence of weak Vander Wall forces between two layers due to these weak forces the two layers are flexible in nature which in result make the Graphite to be used for Lubricating purpose.
Applications:
1-Graphite can be used as the solid lubricant
2-Graphite can be used as a conductor.
3-Graphite can be used as electrodes.