Unit-5
Chemical and electrochemical energy sources
Q1)Classify Fuels.
A1)
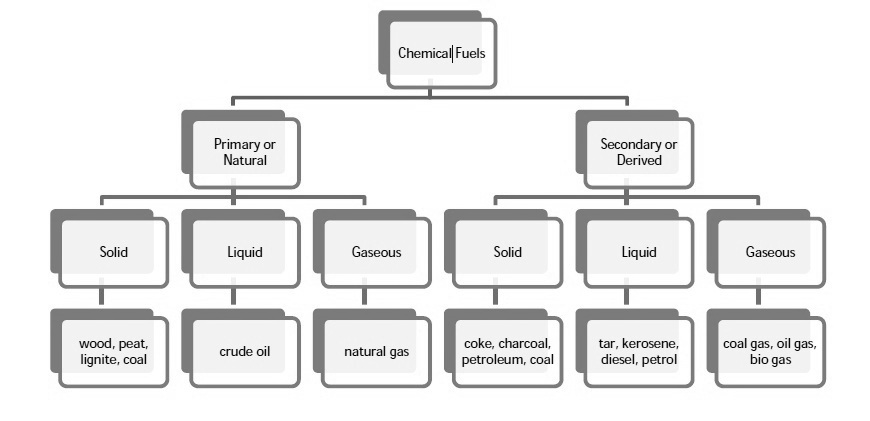
|
Q2)Enlist the characteristics of good fuels.
A2)
1 The fuel should be easily available.
2 It should be dry and should have less moisture content.
3 Dry fuel increases its calorific value.
4 It should be cheap, easily transportable, and has high calorific value.
5 It must have moderate ignition temperature and should leave less ash after combustion.
6 The combustion speed of good fuel should be moderate.
7 It should not burn spontaneously to avoid fire hazards.
8 Its handling should be easy and should not give poisonous gases after combustion.
9 The combustion of good fuel should not be explosive.
Q3)Explain the construction and working of the bomb calorimeter.
A3)
Construction: - a bomb calorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot:-
It is a cylindrical strong stainless-steel pot having a lid. The lid can be fitted with air. Tight to bomb pot by screwing.
- There are two types of electrodes fitted through the lid and there is an oxygen inlet valve as its center.
- One of these electrodes is provided with a ring to hold the crucible containing fuel. There is a thin resistance wire tied to the electrodes in a loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter:-
- There is a stainless steel or copper calorimeter in which the bomb pot is kept. It contains a known volume of water and the water is kept circulating in the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter, which can record the rise in temperature of welter due to absorbing in a heat generated.
- There are insulator stands between the calorimeter and water jacket.
Accessories:-
- There is a pellet press to convert the powder of solid fuel to pellet form. For a liquid fuel, a capsule of negligible weight can be used.
- There is an oxygen cylinder with a pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D. C battery of about 6 volts to start the combustion of fuel.
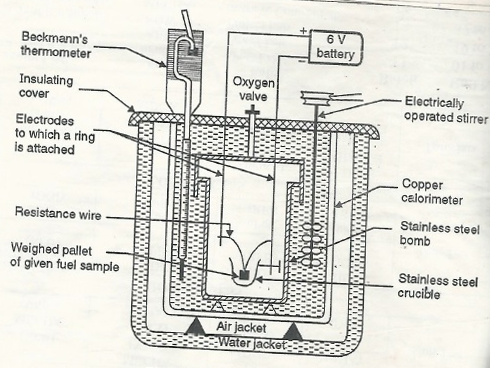 |
Fig: Bomb Caloriemeter
Working:-
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible. Keep the crucible in the ring of the electrode. Keep the resistance wire touching the fuel.
- Add about 10 ml of distilled water at the bottom of the bomb pot and fix the lid tightly to the bomb by screwing.
- Fill the bomb with oxygen at a pressure of about 25 kg / cm².
- Place the bomb in the calorimeter add a known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs. Keep the thermometer and stirrer in the water of the calorimeter.
- Put the plastic cover on the and make electrical connections from the battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water ( t1° c ).
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5. These gases get dissolved in the distilled water in a bomb to form H2SO4 and HNO3 ( along with liberation little heat ).
- Note the maximum temperature reached. After that note, the rate of fall of temperature per minute and the time is taken for reaching to initial temp are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
Q4)Explain the Proximate and ultimate analysis of coal.
A4) There are two methods to analyze coal: ultimate analysis and proximate analysis. The ultimate analysis determines all coal component elements, solid or gaseous and the proximate analysis determines only the fixed carbon, volatile matter, and moisture and ash percentages. The ultimate analysis is determined in a properly equipped laboratory by a skilled chemist, while proximate analysis can be determined with a simple apparatus.
- Measurement of moisture: The determination of moisture content is carried out by placing a sample of powdered raw coal of size 200- micron size in an uncovered crucible, which is placed in the oven kept at 108 +2 °C along with the lid. Then the sample is cooled to room temperature and weighed again. The loss in weight represents moisture.
- Measurement of the volatile matter: A fresh sample of crushed coal is weighed, placed in a covered crucible, and heated in a furnace at 900 + 15 oC. The sample is cooled and weighed. Loss of weight represents moisture and volatile matter. The remainder is coke (fixed carbon and ash).
- Measurement of carbon and ash: The cover from the crucible used in the last test is removed and the crucible is heated over the Bunsen burner until all the carbon is burned. The residue is weighed, which is the incombustible ash. The difference in weight from the previous weighing is the fixed carbon. In actual practice Fixed Carbon or FC is derived by subtracting from 100 the value of moisture, volatile matter, and ash.
- Proximate analysis: The proximate analysis indicates the percentage by weight of fixed carbon, volatiles, ash, and moisture content in coal. The amounts of fixed carbon and volatile combustible matter directly contribute to the heating value of coal. Fixed carbon acts as the main heat generator during burning. High volatile matter content indicates easy ignition of fuel. The ash content is important in the design of the furnace grate, combustion volume, pollution control equipment, and ash handling systems of a furnace.
Fixed carbon: Fixed carbon is the solid fuel left in the furnace after the volatile matter is distilled off. It consists mostly of carbon but also contains some hydrogen, oxygen, sulphur, and nitrogen not driven off with the gases. Fixed carbon gives a rough estimate of the heating value of coal.
Q5) Describe petroleum and its sources.
A5)Petroleum is a naturally occurring liquid found beneath the earth’s surface that can be refined into fuel. Petroleum is a fossil fuel, meaning that it has been created by the decomposition of organic matter over millions of years. Petroleum is formed when large quantities of dead organisms–primarily zooplankton and algae–underneath sedimentary rock are subjected to intense heat and pressure. Sedimentary basins, where ancient sea beds used to lie, are key sources of petroleum. Oil is drilled all over the world. However, three primary sources of crude oil set reference points for ranking and pricing other oil supplies: Brent Crude, West Texas Intermediate, and Dubai and Oman.
Q6) Enlist the composition of petrol.
A6)
Element | Percentage Range |
Carbon | 83 to 85 % |
Hydrogen | 10 to 14 % |
Nitrogen | 0.1 to % |
Oxygen | 0.05 to 1.5 % |
Metals | <0.1 % |
Sulfur | 0.05 to 6.0 % |
Q7)Explain the refining of petroleum.
A7)The separation of miscible liquids into their simpler forms is called fractional distillations. This separation begins when the mixture is heated at a certain temperature where the fractions of the mixture start to vaporize. Crude oil normally contains substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil, and kerosene. The distillation process helps in separating these components effectively.
Crude oil is added to the chamber and is heated with high-pressure steam. The mixture starts boiling and vapor is formed. At this point, various substances enter into the vapor phase. The vapor rises in the fractional distillation column which consists of several plates. The plates have holes that allow the vapor to pass through them. The temperature is usually kept low at the top of the fractionating column. Here, components with the highest boiling point will condense in the lower part of the column while substances with a low boiling point will condense at the top. The condensed vapors or liquid fractions are then removed from the sides of the column. The collected liquid fractions can further be passed through condensers to cool them even more.
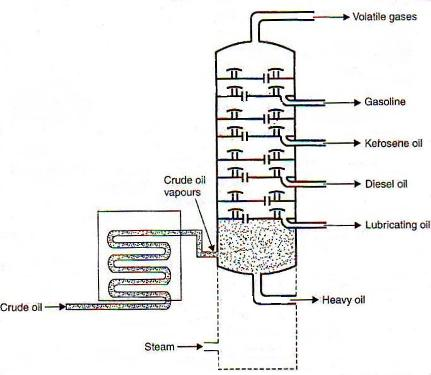 |
Q8)What are the cetane number and octane number?
A8)Cetane number and octane number both are used to measure the tendency of the fuel ignition. The cetane number refers to the eace with which fuel ignites easily at a relatively low temperature. The Research Octane number and Motor Octane number are used in the determination of a single-cylinder test engine in the laboratory while Road Octane Number measures the antiknock performance in an actual vehicle under road driving conditions. Cetane rating indicates the cold starting ability of diesel fuel that is mostly recommended by the automakers of about 45. A high cetane rating means the fuel will ignite easily from heat and pressure and burn quickly.
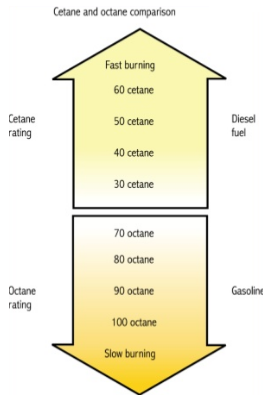
|
Cetane Rating
Fuel | Research Octane Number | Motor Octane Number | Cetane Number | Boiling Point (oC) |
Ethanol | 107 | 89 | 5 | 79 |
Methane (LPG) | 120 | 120 | 0 | -161.66 |
Diesel | -25 | - | 45-55 | 140-360 |
Gasoline | 92-98 | 80-90 | 0-5 | 37-205 |
Q9)What is natural gas?
A9)Methane is the main constituent of natural gas and accounting for about 95% of the total volume. Other components are Ethane, Propane, Butane, Pentane, Nitrogen, Carbon Dioxide, and traces of other gases. Very small amounts of sulphur compounds are also present. Since methane is the largest component of natural gas, generally, properties of methane are used when comparing the properties of natural gas to other fuels. Natural gas is a high calorific value fuel requiring no storage facilities. It mixes with air readily and does not produce smoke or soot. It did not contain sulphur. It is lighter than air and disperses into the air easily in case of a leak.
Q10)Explain the process of electrolysis.
A10)Electrolysis is defined as a process of decomposing ionic compounds into their elements by passing a direct electric current through the compound in a fluid form. The cations are reduced at the cathode and anions are oxidized at the anode. For example, acidified or salt-containing water can be decomposed by passing electric current to their original elements hydrogen and oxygen. Molten sodium chloride can be decomposed into sodium and chlorine atoms.
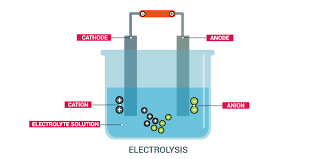 |
Electrolysis is usually done in a vessel named ‘electrolytic cell’ containing two electrodes (cathode and anode) connected to a direct current source and an electrolyte which is an ionic compound undergoing decomposition, in either molten form or a dissolves state in a suitable solvent.
Q11)What are the factors that affect the conductivity of electrolytes?
A11)
1)Nature of electrolyte:- Strong electrolyte ionize completely thus conduct electricity while weak electrolyte ionize partially and hence conduct a small extent of electricity
2)Size of ion and their salvation (mixing with solvent): Greater the size of the ion or salvation less is the conductance.
3)Nature of solvent & viscosity: Electrolyte ionizes more in a polar solvent. The greater the polarity of the solvent, the greater is the ionization, and hence greater is the conductance. Similarly greater the viscosity of the solventless is the conductance.
4)Concentration of the solution: Higher the concentration less is the conductance.