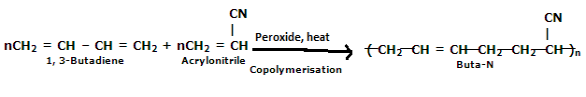|
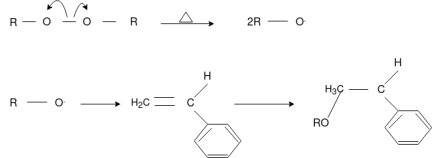
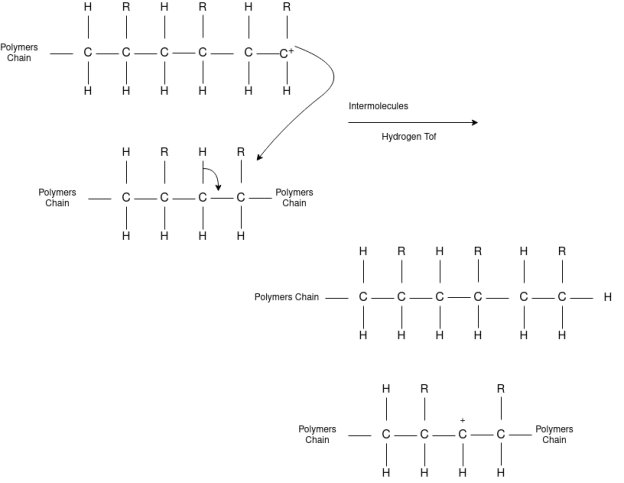
The formation of polymer from the successive addition of free-radical building blocks through the polymerization is called free radical polymerization. In order to obtain a wide variety of different polymers and material composites, free radical polymerization plays a major role in it. The relatively non-specific nature of free-radical chemical interaction makes it one of the most versatile forms of polymerization which allow a facile reaction of polymeric free radical chain ends and other chemical or substrates. The monomers from which addition polymers are made are alkenes. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates, and this is the mechanism followed by most polymerization. The monomers are initiated by traces of oxygen, the pure compounds are stabilized by radical inhibitors.
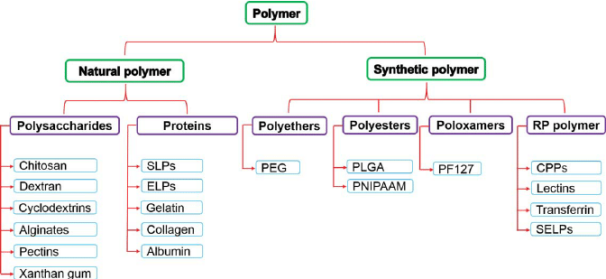
Radical Initiators
Chain Initiation
Chain Propagation
Chain Termination
Chain Transfer Reaction |
Property | Thermoplastics Polymer | Thermosetting Polymer |
Molecular Structure | Linear polymer: weak molecular bonds in a straight-chain formation | Network polymers: high level of crosslinking with strong chemical molecular bonds |
Melting point | Melting point lower than the degradation temperature | Melting point higher than the degradation temperature |
Mechanical | Flexible and elastic. High resistance to impact (10x more than thermosets). Strength comes from crystallinity | Inelastic and brittle. Strong and rigid. Strength comes from crosslinking. |
Polymerization | Addition polymerisation: repolymerised during manufacture (before processing) | Polycondensation polymerisation: polymerised during processing |
Microstructure | Comprised of hard crystalline and elastic amorphous regions in its solid-state | Comprised of thermosetting resin and reinforcing fibre in its solid-state |
Size | Size is expressed by molecular weight | Size is expressed by crosslink density |
Solubility | Can dissolve in organic solvents | Do not dissolve in organic solvents |
Service temperature | Lower continuous use temperature (CUT) than thermosets | Higher CUT than thermoplastics |
- activator (commonly zinc oxide or stearic acid),
- accelerator (guanidines, thiazoles, dithiocarbamates, xanthates, thiurams,
- coagulants (acetic acid, calcium chloride),
- anti-oxidants (amines, phenolics, phosphites),
- color pigments,
- surfactants,
- softeners,
- ant-foaming agents,
- anti-tack agents (Rosin derivates, coumarone-indene resins, aliphatic petroleum resins, alkyl-modified phenol-formaldehyde resins).
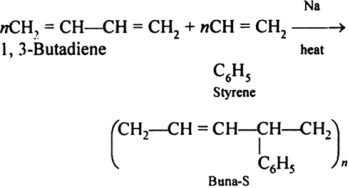
Preparation of Buna-S: Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrene. Buna-S is the replacement of natural rubber while styrene, 2 monomers, and butadiene play a major role in its derivation whereas these 2 monomers are polymerized by two different processes i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene. It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called cold rubber. The obtained rubber is called Styrene-Butadiene Rubber (SBR). Applications: (i) Buna-S is used as the natural rubber i.e., they are widely used in pneumatic tyres. (ii) SBR is extensively used in coated papers. (iii) They are highly used in building materials as a binding material. (iv) SBR is also used as a binder in lithium-ion battery electrodes. |
|