|
1 The fuel should be easily available.
2 It should be dry and should have less moisture content.
3 Dry fuel increases its calorific value.
4 It should be cheap, easily transportable, and has high calorific value.
5 It must have moderate ignition temperature and should leave less ash after combustion.
6 The combustion speed of good fuel should be moderate.
7 It should not burn spontaneously to avoid fire hazards.
8 Its handling should be easy and should not give poisonous gases after combustion.
9 The combustion of good fuel should not be explosive.
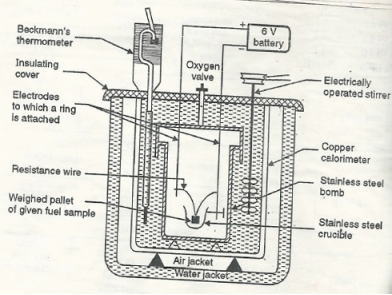
Q3) Explain the construction and working of the bomb calorimeter.A3)Construction: - a bomb calorimeter consists of
|
Element | Percentage Range |
Carbon | 83 to 85 % |
Hydrogen | 10 to 14 % |
Nitrogen | 0.1 to % |
Oxygen | 0.05 to 1.5 % |
Metals | <0.1 % |
Sulfur | 0.05 to 6.0 % |
|
|
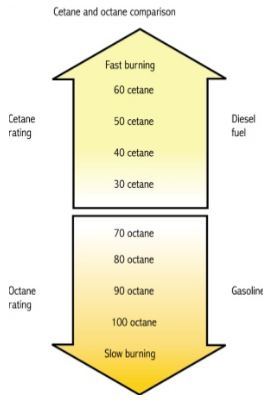
Fuel | Research Octane Number | Motor Octane Number | Cetane Number | Boiling Point (oC) |
Ethanol | 107 | 89 | 5 | 79 |
Methane (LPG) | 120 | 120 | 0 | -161.66 |
Diesel | -25 | - | 45-55 | 140-360 |
Gasoline | 92-98 | 80-90 | 0-5 | 37-205 |
|