UNIT 4
Introduction To Mechanical Engineering
Question 1) Write the applications of first law of thermodynamics study flow process?
Answer 1) Application of first law to study flow process
A steady flow process is one in which matter and energy flow steadily in and out of an open system. In a steady flow process, the properties of the flow remain unchanged with time, that is, the properties are frozen in time. But, the properties need not be the same in all points of the flow. It is very common for a beginner to confuse the term steady with the term equilibrium. But, they are not the same. When a system is at a steady state, the properties at any point in the system are steady in time, but may vary from one point to another point. The temperature at the inlet, for example, may differ from that at the outlet. But, each temperature, whatever its value, remains constant in time in a steady flow process. When a system is at an equilibrium state, the properties are steady in time and uniform in space. By properties being uniform in space, we mean that a property, such as pressure, has the same value at each and every point in the system.
A steady flow is one that remains unchanged with time, and therefore a steady flow has the following characteristics:
i. No property at any given location within the system boundary changes with time. That also means, during an entire steady flow process, the total volume Vs of the system remains a constant, the total mass ms. Of the system remains a constant, and that the total energy content Es of the system remains a constant.
Ii. Since the system remains unchanged with time during a steady flow process, the system boundary also remains the same
Iii. No property at an inlet or at an exit to the open system changes with time. That means that during a steady flow process, the mass flow rate, the energy flow rate, pressure, temperature, specific (or molar) volume, specific (or molar) internal energy, specific (or molar)enthalpy, and the velocity of flow at an inlet or at an exit remain constant.
Iv. Rates at which heat and work are transferred across the boundary of the system remain unchanged.
The application of the first law of thermodynamics to steady-flow processes is defined by two fundamental principles:
(a) Steady mass flow rate m = pAu (mass continuity) where p = fluid density
A = duct area
u = fluid velocity
And m = steady mass flow rate (dm/dt)
(This is usually recast as mv = uA since v = 1/p.)
(b) Conservation of energy (the steady-flow energy equation or SFEE)
Q 1-2 –W 1-2 =m [(h 2 -h 1) + ½ (u 2 2 –u 1 2) + g(Z 2 -Z l )]
Where Z = height above datum.
Note that very often gz is negligible compared with other terms and the reduced SFEE is written as
Q 2 -W 2 =m [(h2 –h1) + ½ (u 2 2 –u 1 2 ) ]
In per unit mass flow rate.
Q/m=q, W/m= w
q 2 - w 2 = (h 2 – h 1) + ½ (u 2 2 –u 1 2 )
An even further reduction by negligible kinetic energy terms here
q2 - w2 = (h2 – h1)
Many processes in reality approximate closely to steady flow, example steady
Conditions in a steam power plant after the start up transient is over and
Steady conditions exist at all points in the system.
Here two essential characteristics of steady flow:
(a) m is constant,
(b) The properties at any station are invariable with time .
The one obvious application of the SFEE where gz is prominent is in a hydroelectric plant where a change in z is essential for power production.
Question 2) Explain difference between isothermal and adiabatic process?
Answer 2) The Difference Between Isothermal and Adiabatic Process
Isothermal process | Adiabatic process |
An isothermal process is defined as one of the thermodynamic processes which occur at a constant temperature | An adiabatic process is defined as one of the thermodynamic processes which occur without any heat transfer between the system and the surrounding |
Work done is due to the change in the net heat content in the system | Work done is due to the change in its internal energy |
The temperature cannot be varied | The temperature can be varied |
There is a transfer of heat | There is no transfer of heat |
Question 3) Explain first law of thermodynamics?
Answer 3) sometimes our body start to sweat and feel warm when we are in a room full of people and the sweating becomes excessive if the room size is small. This happens because our body is trying to cool off hence heat transfers from our body in the form of ‘sweat’. This entails the first law of thermodynamics.
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
According to this law, some of the heat given to system is used to change the internal energy while the rest in doing work by the system. Mathematically,
ΔQ=ΔU+ΔW
Where,
ΔQ = Heat supplied to the system
ΔW= Work done by the system.
ΔU = Change in the internal energy of the system.
If Q is positive, then there is a net heat transfer into the system, if W is positive, then there is work done by the system. So positive Q adds energy to the system and positive W takes energy from the system. It can also be represented as ΔU=ΔQ−W
We can say that internal energy tends to increase when heat is given to the system and vice versa.
Question 4) what is thermodynamics process?
Answer 4) Thermodynamics process represents a transition in which a system changes from one state to another. When the path is completely specified then the change of state is called a process. A Process is defined as the transformation of the system from one fixed state to another fixed state .When any one of the properties changes, the working substance or system is said to have undergone a process.
Some of the processes are identified by special names as given below:
i. Isobaric process (constant pressure process.
Ii. Isochoric process (constant volume process)
Iii. Isothermal process (constant temperature process)
Iv. Isentropic process (constant entropy process)
v. Adiabatic process ( perfectly insulated process)
Question 5) Explain the difference between heat and work?
Answer 5)
WORK HEAT
Interaction | Mechanical | Thermal |
Requires | Force and Displacement | Temperature difference |
Process | Macroscopic pushes and pulls | Microscopic collisions |
Positive value | W > 0 when a gas is compressed. Energy is transferred into system. | Q > 0 when the environment is at a higher temperature than the system. Energy is transferred into system. |
Negative value | W < 0 when a gas expands. Energy is transferred out of system. | Q < 0 when the system is at a higher temperature than the environment. Energy is transferred out of system. |
Equilibrium | A system is in mechanical equilibrium when there is no net force or torque on it. | A system is in thermal equilibrium when it is at the same temperature as the environment. |
Question 6) Explain the limitation of first law of thermodynamics
Answer 6) Limitations of First Law of Thermodynamics
i. The limitation of the first law of thermodynamics is that it does not say anything
About the direction of flow of heat.
Ii. It does not say anything whether the process is a spontaneous process or not.
Iii. The reverse process is not possible. In actual practice, the heat doesn’t
Convert completely into work. If it would have been possible to convert the
Whole heat into work, then we could drive ships across the ocean by extracting
Heat from the water of the ocean.
Question 7) What is kelvin- plank and Clausius statements?
Answer 7)
Kelvin-Plank statement
It is impossible to convert all the heat extracted from a hot body into work. In the heat engine, the working substance takes heat from the hot body, converts a part of it into work and gives the rest to the cold body. There is no engine that can convert all the heat taken from the source into work, without giving any heat into the sink. This means that for obtaining continuous work, a sink is necessary.
Clausius statement
It is not at all possible to transfer heat from a cold body to a hot body without the expenditure of work by an external energy source or its states that the heat energy cannot transfer from a body at a lower temperature to a body at a higher temperature without the addition of energy.
Question 8Write efficiency of nuclear power plant?
Answer 8) The Efficiency of the Nuclear Power Plant
The nuclear power plant efficiency can be decided equally to other heat engines because technically the plant is a large heat engine. The sum of electric power generated for every unit of thermal power will provide the plant is thermal efficiency & because of the thermodynamics second law, there is a higher limit to how efficient these power plants can be.
The normal nuclear power plants attain efficiencies approximately 33 to 37%, equivalent to fossil-fuel plants. High temperature & more current designs such as the Generation IV reactors could acquire above 45% efficiency.
Question 9) Describe types of nuclear power plant?
Answer 9) Types of Nuclear Power Plant
There are two types of nuclear power plants such as pressurized water reactor and boiling water reactor.
Pressurized Water Reactor
In this kind of reactor, regular water is used as a coolant. This is kept at extremely high force so that it does not get a boil. A heat exchanger in this reactor transfers the heated water where the water from the secondary coolant circle is changed into vapour. Therefore, this loop is totally free from the material of radioactive. In this reactor, the coolant water works as a moderator. Because of these benefits, these reactors are used most frequently.
Boiling Water Reactor
In this kind of reactor, a single coolant loop is only available. The water is permissible to heat within the reactor. The steam is produced from the reactor when it heads out from the reactor & the steam will flow throughout the steam turbine. The main drawback of this reactor is, the coolant water approaches the fuel rods & the turbine. So, radioactive material could be located over the turbine.
Question 10) In India, how many nuclear plants are there?
Answer 10) there are seven nuclear plants available in India
1) Kudankulam Nuclear Power Plant, located in Tamil Nadu
2) Tarapur Nuclear Reactor, located in Maharashtra
3) Rajasthan Atomic Power Plant, located in Rajasthan
4) Kaiga Atomic Power Plant, located in Karnataka
5) Kalapakkam Nuclear Power Plant, located in Tamil Nadu
6) Narora Nuclear Reactor, located in Uttar Pradesh
7) Kakarapar Atomic Power Plant, located in Gujarat
Question 11) Explain advantage and disadvantage of nuclear power plant
Answer 11)
Advantages
The advantages of nuclear power plants include the following.
1) It uses less space compared with other power plants
2) It is extremely economical and generates huge electric power.
3) These plants are located near the load center because there is no requirement of huge fuel.
4) It generates a huge amount of power in the process of each nuclear fission
5) It uses less fuel to generate huge energy
6) Its operation is reliable
7) When compared with steam power plants, it is very clean and neat
8) The operating cost is small
9) It doesn’t produce polluting gases
Disadvantages
The disadvantages of nuclear power plants include the following.
1) The cost of primary installation is extremely high when compared with other power stations.
2) The nuclear fuel is expensive so recovering is difficult
3) High capital cost compare with other power plants
4) Technical knowledge is required to operate this plat. So maintenance, as well as salary, will be high.
5) There is a chance of radioactive pollution
6) The response is not efficient
7) The requirement of cooling water is double compare with a steam power plant.
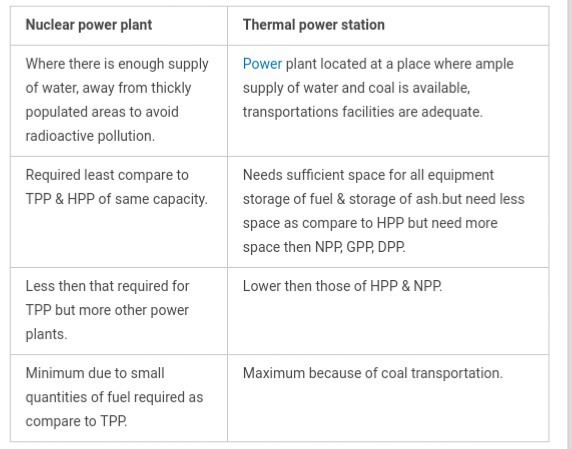
Question 12) Explain the difference between nuclear power plant and thermal power plant?
Answer 12) difference between nuclear power plant and thermal power plant are given below: