CHEM
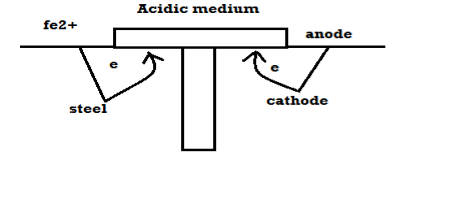
Unit-2Corrosion and Battery Science Q1) Explain the Factors that affect Corrosion?A1) Factors influencing corrosion.A] Nature of metalB] nature of environment Nature of metal: - Position of metal in galvanic series: - When 2 metals are in the contact in the presence of the electrolyte then more active metal undergo corrosion. Greater the difference in their positions, higher is the rate of corrosion. Relative areas of anode and cathode: -If the anode < cathode then rate of corrosion. If the cathode < anode Purity of metal: -Pure metal resists the corrosion Rate of corrosion increases with increases in of impurities which from galvanic cells. Physical state: -Smaller the grain size of metal alloy greater the mate of corrosion. e.g.: - steel get corroded fast than the cost iron because grain size of steel < grain size of cast ion. Nature of oxide film: -If oxide layer – porous Then corrosion take place If oxide layer – nonporous Then no corrosion takes place Over – vtg (over – potential): -To restore position of metal some extra VTG is required is called as over –vtg Higher the over vtg less is the rate of corrosion. e.g.: -Zn, Pb, Cr, Ni, etc. B] nature of environment: -1) temperature: -Higher is temperature higher is the rate of dry corrosion because attacking gas and metal get activated @ higher temp. Higher is the temperature higher is rate of wet corrosion because: - High tempo, according to nearest equation electrode potential is high. 2) moisture: -Higher is the moisture Higher is the rate of dry and wet corrosion 3) PH: -Generally acidic media have more corrosive effect on metals. 4) conductivity of medium: -High the electrical conductivity of aq. conducting medium higher is the rate of corrosion. 5) nature of ions: -Cl -, no3 – have the ability break nonporous oxide layer and cause wet corrosion. Presence of oxalate, m phosphate and silicate ions have the ability to slow down rate of wet corrosion. Q2) Explain the Mechanism of Electrochemical Corrosion?A2) Reaction on anode: -Metal atoms on the surface of anode pass into conducting medium by forming metal ions, leaving behind electrons. M – m+n + ne- Reaction on cathode: -The electrons left on anodic part, flow to the cathodic part depending upon the nature of conducting medium they bring about one of the following reactions: - H2 liberation (H2 Evolution): -If the corroding medium is acidic then H + ions from the medium capture the electrons from the cathode and there is liberation of H2 gas taking place.
Fig: 1 Reaction at the anode and the liberation of electrons and formation of metal ions.
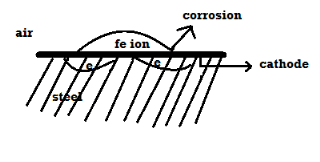
Fig 2: The electrons move to the cathode, depending on the nature of the conducting medium either hydrogen is liberated or oxygen is absorbed b) O2 absorption: -If the corroding medium is neutral or slightly alkaline containing some oxygen gas dissolved the reaction of o2 absorption takes place on the cathode. A) Plating: -If the cathodic part is in the contact with ions of lower plotted metal in the galvanic series, then the ions deposit on the cathode.  + 2e- - cl (atcathode) Q3) What is the Differential Aeration theory of Corrosion?A3) This kind of corrosion occurs when the oxygen concentration differs across a metal surface. This difference in oxygen concentration creates an anode and cathode on the metal surface, subsequently oxidation occurs on the metal surface. In the differential aeration the portion that receives the high concentration forms the cathode and the portion that receives lesser oxygen forms the anode. Resulting in the corrosion of the metal surface that has less oxygen concentration.Some examples, where there may be differences in oxygen concentration are found in crevices, cracks and certain joints, differential corrosion also occurs in metals that are partially immersed in water as the concentration in water is different from the concentration present in the atmosphere.
+ 2e- - cl (atcathode) Q3) What is the Differential Aeration theory of Corrosion?A3) This kind of corrosion occurs when the oxygen concentration differs across a metal surface. This difference in oxygen concentration creates an anode and cathode on the metal surface, subsequently oxidation occurs on the metal surface. In the differential aeration the portion that receives the high concentration forms the cathode and the portion that receives lesser oxygen forms the anode. Resulting in the corrosion of the metal surface that has less oxygen concentration.Some examples, where there may be differences in oxygen concentration are found in crevices, cracks and certain joints, differential corrosion also occurs in metals that are partially immersed in water as the concentration in water is different from the concentration present in the atmosphere.
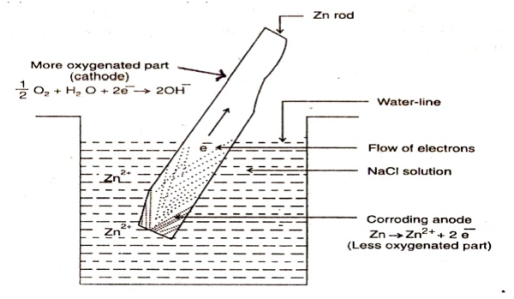
Fig 3: the figure shows a metal rod partially dipped in a conducting medium, the part of the metal above the surface is more aerated and forms the cathode, the less aerated part inside the medium forms the anode and gets corroded. Q4) Differentiate between Pitting and Stress Corrosion?A4) Pitting corrosion, or pitting, is a localised corrosion, where small holes are formed on the metal surface, in this small area depassivation occurs that becomes anodic and the rest of the vast area becomes the cathode, oxidation occurs at the anode and reduction at the cathode, leading to a localised galvanic corrosion. The corrosion penetrates the mass of the metal, with a limited diffusion of ions.Stress CorrosionThis corrosion is characterised by cracks proliferating either intergranular or Tran granularly, there are many types of stress corrosion for example corrosion that is chloride induced or H2S induced corrosion.Stress corrosion occurs due to three factorsTensile stress in the material A corrosion medium: especially chloride -bearing or hydrogen sulphide media. Chloride-induced stress corrosion normally occurs above 600C The material used is susceptible to stress corrosion. A precursor of stress corrosion cracking in chloride-bearing environment is pitting corrosion, occurring if the stainless steel is not sufficiently resistant to pitting Q5) Explain three methods to Prevent corrosion? A5) The three methods area) Design and selection of material A good selection of material and a proper design are very effective in reducing the cost of corrosion and also attain low-cost reliability.In plenty of structures, the absence of a good design is considered as the weakest link in the control of corrosion. The design should possess good mechanical properties and strength, to confirm that the structure is resistant to corrosion. A strong knowledge of the corrosion process is also required.The key to control corrosion is the material corrosion, but a proper design should also not be neglected, the selection and design is equally important in corrosion control. In many structures, a lack of design consideration given to corrosion control is the weakest link in corrosion control. b) Cathodic and Anodic Protection Cathodic Protection
Cathodic protection in this process the main principle is to switch from the works by changing over undesirable anodic (active) sites on to a metal's surface to cathodic (passive) destinations in the presence of a restricting current. This restricting current provides free electrons and also supplies power to the nearby anodes,
Cathodic protection can, the presence of galvanic anodes. This process is also known as conciliatory form, this technique utilises metal anodes together with the electrolytic condition, to make themselves strong (erode) so as to secure the cathode. This technique proves to save the metals galvanic corrosion.
Though the metal that requires protection can differ, the conciliatory anodes are largely made up of magnesium, or zinc, metals that have the most negative electro-potential. The arrangement of galvanic setup gives a glimpse of the distinctive electro-potential - or integrity - of metals and amalgams.
In a conciliatory framework, the anode should be supplanted regularly as the metallic particles move from the anode to the cathode, which drives the anode to erode more rapidly. The second technique for cathodic security is alluded to as awed current protection.
This strategy, which is regularly used to ensure covered pipelines and ship bodies, requires an elective wellspring of direct electrical current to be provided to the electrolyte.
Sacrificial AnodeIt is a method where an easily eroded material is deliberately placed in a tank or a pipe to be sacrificed for corrosion and the rest of the system remains free from corrosion.A sacrificial anode is also known as a galvanic anodeThe mechanism that occurs in this process is very similar to that of an electrochemical system. In this technique the metal that is protected is placed on the cathode side and then a more reactive metal or alloy (having a larger potential difference than the protected metal) is chosen and connected to the protected metal as an anode. The redox reaction will proceed spontaneously. As the reaction proceeds the sacrificial metal gets consumed as oxidation reaction occurs at the anode, simultaneously reduction reaction occurs on the cathode, thus preventing the metal from erosion. Thus, corrosion on the protected metal is successfully shifted to the anode, protecting the metal.Sacrificial anodes are normally supplied with either lead wires or cast-m straps to facilitate their connection to the structure being protected. The lead wires may be attached to the structure by welding or mechanical connThe materials used for sacrificial anodes are either relatively pure active metals, such as zinc or magnesium, or are magnesium or aluminium alloys that have been specifically developed for use as sacrificial anodes.Advantages of using sacrificial anodes:Can be used where there is no power Lower initial cost Less supervision required Comparatively simple installation and additional anodes can easily be added if the initial installation proves to be inadequate Sacrificial anodes are used to protect:Hulls of ships Water heaters Pipelines Distribution systems Above-ground tanks Underground tanks Refineries The anodes in sacrificial anode cathodic protection systems must be periodically inspected and replaced when consumed. c)Protective surface coatingThis is the most economical method and simple wherein the membrane surface is coated by a layer. This method can be carried out on a large scale in industries.The most common coating materials include tin, lead, aluminium etc. The principle in this method includes formation of a transition layer from the various alloy compositions. To the substrate inter-metallic compounds of the two metals are normally present and on the exterior are alloys that consist of solid solution alloys that predominantly form the coating materials. Surface coating is a process where a substance is applied to other materials bringing about changes like gloss, colour, resistance to chemical attacks or permeability, however the bulk properties of the material remain unchanged. Production of surface coating by any method depends primarily on two factors: the cohesion between the film forming substances and the adhesion between the film and the substrate. The development of science and technology revolutionized the surface coating industry Q6) Explain the Types of Corrosion?A6) Chemical corrosion: The direct reaction of Atmospheric gases (like halogens, oxygen, oxides of nitrogen and sulphur, fumes of chemicals and with metals and hydrogen sulphide), on the surface of the metal causes corrosion. Oxygen is the most responsible for corrosion of metals than any other gases or chemicals. This process occurs in the absence of moisture and affects the metal surface directly. Corrosion products are formed at the site of corrosion.Electrochemical Corrosion: This corrosion occurs in metals that come in direct contact with a conducting liquid of two different metals are dissolved in a solution partly. There is formation of galvanic cell on the metal, as part of the metal acts as an anode and the rest is the cathode, the chemical in the solution along with the humidity acts as the electrolyte. Oxidation takes place in such conditions resulting in corrosion at the anode surface and reduction occurs at the cathode surface of the metal. In this case corrosion occurs at the anode but rust gets deposited on the cathode. Q7) Write a note on Alkaline Fluid Cell?A7) The Alkaline Fuel cells, have a solution of potassium hydroxide in water as an electrolyte, these are one of the most efficient types of fuel cells and have about 60% efficiency in space applications, the alkaline fuel cell (AFC)is also called as the Bacon fuel cell, named after the inventor. These cells are one of the most extensively used cells, which NASA is using since 1960s. The Alkaline fuel cells are very vulnerable to contamination; therefore, they require hydrogen and oxygen in its pure form, this property makes the AFC limit their application and expensive to operate. The electrolyte is a solution of potassium hydroxide in water, the cathode and anode are made up of low cost and non precious metals like nickel. The Potassium hydroxide ionizes to form potassium ions(K+) and (OH-) ions. The hydrogen gas is oxidised to hydrogen ions and combines with the hydroxyl ions, resulting in the formation of water (H2O), with the release of two electrons. The electrons that are released flow through the external circuit and return to the cathode, where the electrons reduce oxygen to form water and hydroxide ions. The excess heat is removed as a by-product from the fuel cell, the heat produced is hot enough to provide steam to power a steam turbine. Buildings can also be heated using this heat. The main disadvantage of the AFC is that hydrogen and Oxygen have to be in pure form and to be supplied continuously. The cell life time is easily affected by carbon dioxide as it poisons the fuel cell.The AFC are less cost effective as the cannot be operated above 8000 hours.
Fig 5: Alkaline fuel cells Q8) Write the Applications and Advantages of Fuel cells?
A8) Fuel cell technology has a wide range of applications, a lot of research is going on about the manufacture of a cost-efficient automobile which is powered by a fuel cell. A few applications areFuel cells are used in electrical vehicles, they are more eco-friendly than the internal combustion engine-based vehicles. Fuel calls are utilised to power space expeditions which also includes appolo space program. In general, the by-products of the fuel cells are heat and water. Fuel cell portability is extensively used in military applications. Several electronic devices are powered by the fuel cells. In many remote areas, fuel cells are used as primary or backup sources of electricity. Advantages:
High Efficiency-Fuel cells achieve over 80% of energy efficiency when used for co-generation. Good reliability- The quality of power produced by the fuel cells does not degrade over time. Noise- Compared to the conventional energy production, these cells offer a more silent and smoother substitute. Environmentally beneficial-they reduce the CO2 and harmful pollutant emission to a large extent. Size reduction- the fuel cells are substantially lighter and largely compact. Q9) What are Nickel-Cadmium cells?A9) A nickel-cadmium is a secondary cell that possess two plates. The active material of the positive plate (anode) is Ni(OH)4 and the negative plate (cathode) is of cadmium (Cd) when fully charged. Potassium hydroxide (KOH) forms the electrolyte, a small quantity of lithium hydrate is added to increase the capacity and life of battery. Many cells are connected in series to get the voltage output, as the voltage produces by a single cell is very low. This arrangement is therefore called nickel-cadmium battery.
Fig 4: Nickel Cadmium BatteryIn these batteries, the container of this battery is electrically connected to positive plates, the positive plate number is one more than the negative plate.Nickel Cadmium Battery Working PrincipleWhen the cell is fully charged, its positive plate is of Ni(OH)4 and its negative plate is of cadmium (Cd).
Discharging: When the cell discharges, the potassium hydroxide (KOH) is dissociated into potassium (K+) and hydroxyl (OH–) ions.
The hydroxyl ions move towards the cathode and potassium ions move to the anode. The following chemical reaction takes place during discharging.
At cathode: Cd + 2OH —–> Cd(OH)2
At anode: Ni(OH)4 + 2K ——> 2KOH + Ni(OH)2
Thus, the anode is converted from Ni(OH)4 to NI(OH)2 and cathode is converted from cadmium (Cd) to cadmium hydroxide [Cd(OH)2]. The strength of the electrolyte remains the same.
Charging: When the battery is put on charging, the hydroxyl (OH-) ions move towards the anode, whereas the potassium ions (K+) move towards the cathode. The following chemical reaction takes place during the charging:
At anode: Ni(OH)2 + 2OH —–> NI(OH)4
At cathode: Cd(OH)2 + 2K —–> Cd + 2KOH
The anode and cathode thus regain their chemical composition, and the strength of the electrolyte is unchanged. Q10) What is the Pilling-Bedworth Rule?A10) This rule gives an idea about nature of oxide film, whether it is porous or nonporous. The rule states that, Metal ‘if the volume of oxide < volume of metal consumedThen, Oxide layer – porousElse, oxide layer – nonporous PBR = If, PBR > 1 but PBR < 1.45 Metal oxide layer – nonporous(protective)
If, PBR > 1 but PBR < 1.45 Metal oxide layer – nonporous(protective)
|
|
 + 2e- - cl (atcathode) Q3) What is the Differential Aeration theory of Corrosion?A3) This kind of corrosion occurs when the oxygen concentration differs across a metal surface. This difference in oxygen concentration creates an anode and cathode on the metal surface, subsequently oxidation occurs on the metal surface. In the differential aeration the portion that receives the high concentration forms the cathode and the portion that receives lesser oxygen forms the anode. Resulting in the corrosion of the metal surface that has less oxygen concentration.Some examples, where there may be differences in oxygen concentration are found in crevices, cracks and certain joints, differential corrosion also occurs in metals that are partially immersed in water as the concentration in water is different from the concentration present in the atmosphere.
+ 2e- - cl (atcathode) Q3) What is the Differential Aeration theory of Corrosion?A3) This kind of corrosion occurs when the oxygen concentration differs across a metal surface. This difference in oxygen concentration creates an anode and cathode on the metal surface, subsequently oxidation occurs on the metal surface. In the differential aeration the portion that receives the high concentration forms the cathode and the portion that receives lesser oxygen forms the anode. Resulting in the corrosion of the metal surface that has less oxygen concentration.Some examples, where there may be differences in oxygen concentration are found in crevices, cracks and certain joints, differential corrosion also occurs in metals that are partially immersed in water as the concentration in water is different from the concentration present in the atmosphere.
|
Cathodic protection in this process the main principle is to switch from the works by changing over undesirable anodic (active) sites on to a metal's surface to cathodic (passive) destinations in the presence of a restricting current. This restricting current provides free electrons and also supplies power to the nearby anodes,
Cathodic protection can, the presence of galvanic anodes. This process is also known as conciliatory form, this technique utilises metal anodes together with the electrolytic condition, to make themselves strong (erode) so as to secure the cathode. This technique proves to save the metals galvanic corrosion.
Though the metal that requires protection can differ, the conciliatory anodes are largely made up of magnesium, or zinc, metals that have the most negative electro-potential. The arrangement of galvanic setup gives a glimpse of the distinctive electro-potential - or integrity - of metals and amalgams.
In a conciliatory framework, the anode should be supplanted regularly as the metallic particles move from the anode to the cathode, which drives the anode to erode more rapidly. The second technique for cathodic security is alluded to as awed current protection.
This strategy, which is regularly used to ensure covered pipelines and ship bodies, requires an elective wellspring of direct electrical current to be provided to the electrolyte.
Sacrificial AnodeIt is a method where an easily eroded material is deliberately placed in a tank or a pipe to be sacrificed for corrosion and the rest of the system remains free from corrosion.A sacrificial anode is also known as a galvanic anodeThe mechanism that occurs in this process is very similar to that of an electrochemical system. In this technique the metal that is protected is placed on the cathode side and then a more reactive metal or alloy (having a larger potential difference than the protected metal) is chosen and connected to the protected metal as an anode. The redox reaction will proceed spontaneously. As the reaction proceeds the sacrificial metal gets consumed as oxidation reaction occurs at the anode, simultaneously reduction reaction occurs on the cathode, thus preventing the metal from erosion. Thus, corrosion on the protected metal is successfully shifted to the anode, protecting the metal.Sacrificial anodes are normally supplied with either lead wires or cast-m straps to facilitate their connection to the structure being protected. The lead wires may be attached to the structure by welding or mechanical connThe materials used for sacrificial anodes are either relatively pure active metals, such as zinc or magnesium, or are magnesium or aluminium alloys that have been specifically developed for use as sacrificial anodes.Advantages of using sacrificial anodes:
|
A8) Fuel cell technology has a wide range of applications, a lot of research is going on about the manufacture of a cost-efficient automobile which is powered by a fuel cell. A few applications are
|
Discharging: When the cell discharges, the potassium hydroxide (KOH) is dissociated into potassium (K+) and hydroxyl (OH–) ions.
The hydroxyl ions move towards the cathode and potassium ions move to the anode. The following chemical reaction takes place during discharging.
At cathode: Cd + 2OH —–> Cd(OH)2
At anode: Ni(OH)4 + 2K ——> 2KOH + Ni(OH)2
Thus, the anode is converted from Ni(OH)4 to NI(OH)2 and cathode is converted from cadmium (Cd) to cadmium hydroxide [Cd(OH)2]. The strength of the electrolyte remains the same.
Charging: When the battery is put on charging, the hydroxyl (OH-) ions move towards the anode, whereas the potassium ions (K+) move towards the cathode. The following chemical reaction takes place during the charging:
At anode: Ni(OH)2 + 2OH —–> NI(OH)4
At cathode: Cd(OH)2 + 2K —–> Cd + 2KOH
The anode and cathode thus regain their chemical composition, and the strength of the electrolyte is unchanged. Q10) What is the Pilling-Bedworth Rule?A10) This rule gives an idea about nature of oxide film, whether it is porous or nonporous. The rule states that, Metal ‘if the volume of oxide < volume of metal consumedThen, Oxide layer – porousElse, oxide layer – nonporous PBR =
 If, PBR > 1 but PBR < 1.45 Metal oxide layer – nonporous(protective)
If, PBR > 1 but PBR < 1.45 Metal oxide layer – nonporous(protective)0 matching results found