Unit 4
Question Bank
Question 1) Explain the Definition of Steam Terms?
Answer 1)
Heat – A form of energy
Temperature – The degree of hotness with no implication of amount of heat energy. Expressed in terms of degrees Fahrenheit: 32° is freezing, 212° is the boiling point at atmospheric pressure.
BTU - (British thermal unit) The amount of heat energy required to raise the temperature of one pound of water 1°. More correctly, 1/180 of the amount of heat required to raise 1 pound of water from 32° to 212° at atmospheric pressure.
Pressure - The collision of molecules of a gas with the walls of a container, represented in PSI, PSIA, or PSIG.
PSI: Pounds per square inch
PSIA: Pounds per square inch absolute - includes atmospheric pressure (0 PSIG=14.7 PSIA)
PSIG: Pounds per square inch gauge – measures pressure above atmospheric
Sensible Heat – Expressed in BTU/lb. The heat required to bring one lb. Of water from the freezing point to boiling point corresponding to any pressure. Higher pressures mean higher boiling points.
Latent Heat of Evaporation – Expressed in BTU/lb. The amount of heat required to convert one pound of water to steam. Latent heat is the potential energy of steam or the useable part of steam. When these BTUs are released or given up, condensation takes place and a pound of condensate results. Note that as pressure increases, the BTU requirement to change one pound of water to steam decreases.
Specific Volume – Expressed in ft3/lb. The space occupied by one pound of steam at a particular pressure. As pressure increases, specific volume decreases
Question 2) Explain the Types of Steam?
Answer 2) Steam is an invisible gas created by adding heat energy to water. It is liquid water changed to its gaseous state.
Saturated steam - steam in immediate contact with the water from which it is being generated. If the pressure remains constant, any loss of heat or BTUs will result in condensation.
Superheated steam – If more heat is added to dry saturated steam at a constant pressure, increasing its temperature and specific volume, super-heated steam is produced. Heat must be lost, and temperature reduced before condensation occurs.
Flash steam - when condensate, at saturation temperature and pressure, is discharged into a region of lower pressure, it automatically adjusts to the saturated conditions at the lower pressure. In effect, some of the condensate is “re-evaporated” into steam.
Question 3) What do you mean by the term state in thermodynamics?
Answer 3)
The application of thermodynamic principles begins by defining a system that is in some sense distinct from its surroundings. For example, the system could be a sample of gas inside a cylinder with a movable piston, an entire steam engine, a marathon runner, the planet Earth, a neutron star, a black hole, or even the entire universe. In general, systems are free to exchange heat, work, and other forms of energy with their surroundings.
A system’s condition at any given time is called its thermodynamic state. For a gas in a cylinder with a movable piston, the state of the system is identified by the temperature, pressure, and volume of the gas. These properties are characteristic parameters that have definite values at each state and are independent of the way in which the system arrived at that state. In other words, any change in value of a property depends only on the initial and final states of the system, not on the path followed by the system from one state to another. Such properties are called state functions. In contrast, the work done as the piston moves and the gas expands and the heat the gas absorbs from its surroundings depend on the detailed way in which the expansion occurs.
The behaviour of a complex thermodynamic system, such as Earth’s atmosphere, can be understood by first applying the principles of states and properties to its component part in this case, water, water vapour, and the various gases making up the atmosphere. By isolating samples of material whose states and properties can be controlled and manipulated, properties and their interrelations can be studied as the system changes from state to state.
Question 4) Explain the difference between heat and work?
Answer 4)
WORKHEAT
Interaction | Mechanical | Thermal |
Requires | Force and Displacement | Temperature difference |
Process | Macroscopic pushes and pulls | Microscopic collisions |
Positive value | W > 0 when a gas is compressed. Energy is transferred into system. | Q > 0 when the environment is at a higher temperature than the system. Energy is transferred into system. |
Negative value | W < 0 when a gas expands. Energy is transferred out of system. | Q < 0 when the system is at a higher temperature than the environment. Energy is transferred out of system. |
Equilibrium | A system is in mechanical equilibrium when there is no net force or torque on it. | A system is in thermal equilibrium when it is at the same temperature as the environment. |
Question 5) Explain Quality of steam (Dryness fraction)?
Answer 5)
Dryness fraction in simple words denotes the mass of dry steam in given steam. Or how much steam is dry or in other words how much water vapour is present in steam. It is denoted by ‘x’.
X = M / M+m
Where M=mass of the dry steam
m=mass of water vapour
The use of dryness fraction allows us to know both the mass of dry steam and mass of water vapour.
Now, see
If x = 0.9 that means dry steam is 0.9 kg and water vapour is 0.1 kg in 1 kg of given steam.
Obviously for dry steam, x = 1
Quality is represented in percentage but meaning is same as ‘x’.
If quality of steam is 80%, then it has 80% of dry steam and 20 % water vapour by mass.
Question 6) Explain Specific volume?
Answer 6)
Gases (steam is a gas) occupy less space under higher pressure than under lower pressure. This means 1 kilogram of steam occupies different volumes, depending upon its pressure. The term specific volume refers to the volume that one kg of steam occupies at a given pressure and temperature.
Unit is m3 / kg denoted by v
Question 7) Explain the types of steam?
Answer 7)
Steam is an invisible gas created by adding heat energy to water. It is liquid water changed to its gaseous state.
Saturated steam - steam in immediate contact with the water from which it is being generated. If the pressure remains constant, any loss of heat or BTUs will result in condensation.
Superheated steam – If more heat is added to dry saturated steam at a constant pressure, increasing its temperature and specific volume, super-heated steam is produced. Heat must be lost, and temperature reduced before condensation occurs.
Flash steam - when condensate, at saturation temperature and pressure, is discharged into a region of lower pressure, it automatically adjusts to the saturated conditions at the lower pressure. In effect, some of the condensate is “re-evaporated” into steam.
Question 8) Explain steam properties?
Answer 8) Steam properties:
When we provide continuous heat to water then at 100 temperature and 1 atm pressure, it boils and changes its phase from liquid to vapour. This vapour is known as steam.
Steam contains more energy as it has both sensible heat and latent heat of vaporization. Steam has been a popular mode of conveying energy since the industrial revolution. Steam issued for generating power and also used in process industries such as sugar, paper, fertilizer, refineries, petrochemicals, chemical, food, synthetic fibre and textiles the following characteristics of steam make it so popular and useful to the industry:
• Highest specific heat and latent heat
• Highest heat transfer coefficient
• Easy to control and distribute
• Cheap and inert
Types: Wet steam, Dry steam, Superheated steam
Wet Steam: When steam contains water particles then it is known as Wet steam
Question 9) Explain Indirect Efficiency?
Answer 9)
The indirect efficiency of a boiler is calculated by finding out the individual losses taking place in a boiler and then subtracting the sum from 100%. This method involves finding out the magnitudes of all the measurable losses taking place in a boiler by separate measurements. All these losses are added and subtracted from 100% to find out the final efficiency. Blow down valve is kept closed during the procedure. This method should be implemented as per the norms provided in BS845 standards. The losses calculated include stack losses, radiation losses, blow down losses etc.
Question 10) How to Use the Steam Table?
Answer 10)
In steam tables the properties of the dry steam are listed and for the wet steam the properties may be calculated from the steam tables of the dry and saturated steam.
For values that are not listed exactly in the tables, the value between two figures can be obtained by linear interpolation. Interpolation is a mathematical tool by which, depending on the interval between two variables, a value in between can be calculated.
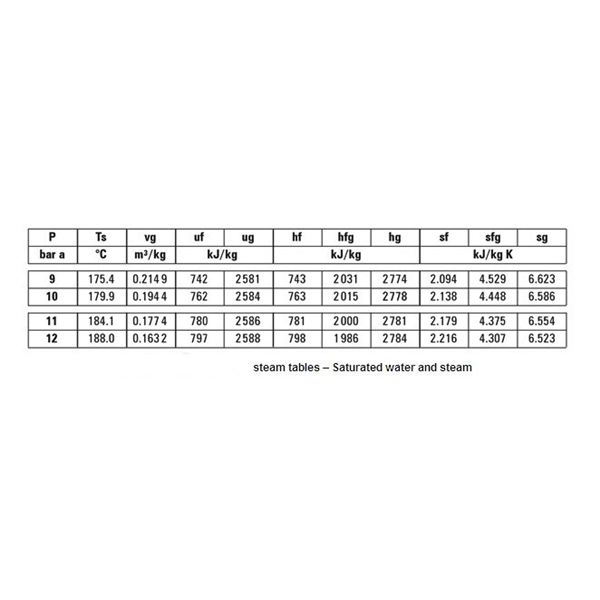 The steam table shown above is a saturated water and steam table. As all the other tables are used on the same principle we will only discuss this one. For an absolute pressure of 9 bars, the saturation temperature is 175.4 C. It means that at a temperature of 175.4 and above C all of the steam will be saturated. Of course, any temperature above this will be super heating of the steam.
The steam table shown above is a saturated water and steam table. As all the other tables are used on the same principle we will only discuss this one. For an absolute pressure of 9 bars, the saturation temperature is 175.4 C. It means that at a temperature of 175.4 and above C all of the steam will be saturated. Of course, any temperature above this will be super heating of the steam.
It must be noted however that at 175.4 C, depending on the latent heat supplied for vaporization, the steam can have any dryness faction. Vg is the specific volume of steam, hf is the specific enthalpy of water, hg is the specific enthalpy of steam, sf is the specific entropy of water, and sg is the specific entropy of steam.
We will now become familiar with this formula:
h = hf + xL
Where x is dryness fraction and L = hg – hf
By the above formula, if we know the dryness fraction of steam, we can calculate the enthalpy of wet steam, and its value would lie between that of the saturated water and saturated steam.
For example if the dryness fraction is 0.8 for steam at 9 bars absolute pressure in bars. Referring to the steam table above, hf = 743 kJ/Kg, L = 2031 kJ/Kg,
h = 743 + 0.8 x 2031 = 2367.8 kJ/Kg