Unit 08
Analytical Techniques
Q-1Explain about the measurement of pH.
The pH of a solution is the negative logarithm (base 10) of the activity of the hydrogen ions (H+) in the solution. In solutions of low ionic strength, pH can be defined as the negative logarithm of the molar concentration of the hydrogen ions because activity and concentration are nearly identical in these solutions. One method for determining pH is by use of a chemical acid-base indicator, which consists of a dye that is either a weak acid or a weak base. The dye has one color in its acidic form and a second color in its basic form. Because different dyes change from the acidic to the basic form at different pH values, it is possible to use a series of dyes to determine the pH of a solution. A small portion of the dye or dye mixture is added to the analyte or a portion of the analyte is added to the dye mixture (often on a piece of paper that is permeated with the indicator). By comparing the color of the indicator or indicator mixture that is in contact with the sample to the colors of the dyes in their acidic and basic forms, it becomes possible to determine the pH of the solution.
Q-2What are conductance?
Conductance is the measure of how easily electricity flows along a certain path through an electrical element, and since electricity is so often explained in terms of opposites, conductance is considered the opposite of resistance. In terms of resistance and conductance, the reciprocal relationship between the two can be expressed through the following equation:
R = 1/G,
G=1/R;
Where,
R = resistance
G = conduction
Q-3Explain spectroscopic techniques.
Spectroscopic techniques have been applied in virtually all technical fields of science and technology. Radio frequency spectroscopy of nuclei in a magnetic field has been employed in a medical technique called Magnetic Resonance Imaging (MRI) to visualize the internal soft tissue of the body with unprecedented resolution. Microwave Spectroscopy was used to discover the so-called three-degree black body radiation, the remnant of the big bang (i.e., the primeval explosion) from which the universe is thought to have originated. The internal structure of the proton and neutron and the state of the early universe up to the first thousandth of a second of its existence are being unraveled with spectroscopic techniques using high-energy particle accelerators. The constituents of distant stars, intergalactic molecules, and even the primordial abundance of the elements before the formation of the first stars can be determined by optical, radio, and X-Ray spectroscopy. Optical spectroscopy is used routinely to identify the chemical composition of matter and to determine its physical structure.
Q-4What is selection rule?
On the irradiation of the sample with infrared electromagnetic radiation then its frequency can be measured by the Infrared spectroscopy. It is a technique used to study the vibrations between atoms because atomic vibrational excitations occur in the infrared region of the electromagnetic spectrum. The bonds between atoms can be thought of as a spring connecting two masses. In the spring-mass analogy the moving system can be approximated by a simple harmonic oscillator. The frequency oscillation is proportional to
√k/m
Where,
k is the spring constant
m is the mass of the object.
Q-5What are monochromator?
Monochromator used to measure the wavelength of the light. Monochromator is placed between the sample and the source of light passing from that the light passes to the detector. Here the monochromatic used to detect the wavelength of the light form the source. While on moving further there is a Double beam Ultraviolet-Visible instrument is used here is a splitter and a series of mirrors to get the beam to a reference sample and the sample to be analyzed, this allows for more accurate readings. In the simultaneous Ultraviolet-Visible instruments monochromator is removed hence it has diode array detectors that allow the instrument to simultaneously detect the absorbance at all wavelengths. This instrument is much faster and accurate than double and single Ultraviolet-Visible instrument.
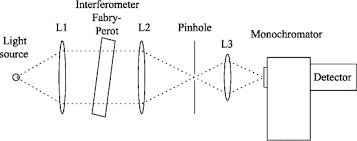
Q-6Explain UV-Visible spectroscopy?
Ultraviolet and Visible Spectroscopy helps ion the determination of the absorbance spectra of the compound in the solution. The absorbance of light energy or the electromagnetic radiations are observed. This is responsible for the exciting the electron from the ground state to the first singlet excited state of a compound. The UV-Visible region of energy for the electromagnetic spectrum covers 1.5 - 6.2 eV which relates to a wavelength range of 800 - 200 nm.
Q-7Explain the applications of UV-Visible Spectroscopy.
1- This is majorly used in the analytical chemistry.
2- This can be used as a detector for the High-performance liquid chromatography.
Ultraviolet-Visible Spectroscopy is used as the semiconductor in the industry to measure the thickness and optical properties of wafer.
Q-8Explain rotational spectroscopy.
Rotational Spectroscopy is also called as the microwave spectroscopy. It is the measurement of energy of transition between quantized rotational states of molecules in the gas phase. The rotational spectra of non-polar molecules cannot be observed by this method while it can be measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. The molecules of the Rotational spectroscopy are classified according to their symmetry. The important feature of molecular rotational spectroscopy for chemical analysis is that the resolution of the spectral transitions is sufficiently high that the spectra of isomers with only small changes in mass distribution can be measured without spectral overlap. For example, the 13C- isotopologues of molecules, isomers created when a single carbon atom is isotopically substituted, are routinely resolved in the instruments used for rotational spectroscopy. The extreme sensitivity changes to in the mass distribution make rotational spectroscopy well-suited to distinguishing diaestromers of molecules with multiple chiral centers. A second advantage of the high-resolution of the spectrometers is that complex sample mixtures can be analyzed directly without spectral overlap. An example of the rotational spectrum from the vapor head space of an essential oil will be presented later to illustrate this capability.
Q-9Enlist the applications of UV-Visible spectroscopy.
1- Rotational Spectroscopy is used in the determination of molecular structure in the gas phase molecule.
2- Rotational Spectroscopy also provide the information about the electronic structures of molecule.
This plays the major role in exploration of chemical composition of interstellar medium.
Q-10Explain infrared spectroscopy.
The change in the vibrational motion of molecule is absorbed in the Infrared Region this is why it is also called as the Infrared Spectroscopy. If vibration in the molecule persists then it should change its dipole moment that may be the magnitude form or oriented with its direction. When taking the example of Polar molecule (A-B) as the polar molecule shows the property of the high electronegative atom attracts the electron pair towards it so that it gets partial negative and partial positive charges. So, in the polar molecule the atoms are making rotation therefore one side is positive pole while other is negative but due to the rotation at its own axis then there is no change in the magnitude effects hence it posses the generation of magnetic field which in result there in the electric flux or there is a change in the direction.
Q-11Enlist the applications of Infrared spectroscopy.
1-Infrared technique is used in organic and inorganic chemistry.
2-This is used in quality control, dynamic measurement.
3-This is majorly used in the forensic labs.
4-Infrared Spectroscopy is used in measuring the degree of polymerization in manufacturing the polymers.
Unit 08
Analytical Techniques
Q-1Explain about the measurement of pH.
The pH of a solution is the negative logarithm (base 10) of the activity of the hydrogen ions (H+) in the solution. In solutions of low ionic strength, pH can be defined as the negative logarithm of the molar concentration of the hydrogen ions because activity and concentration are nearly identical in these solutions. One method for determining pH is by use of a chemical acid-base indicator, which consists of a dye that is either a weak acid or a weak base. The dye has one color in its acidic form and a second color in its basic form. Because different dyes change from the acidic to the basic form at different pH values, it is possible to use a series of dyes to determine the pH of a solution. A small portion of the dye or dye mixture is added to the analyte or a portion of the analyte is added to the dye mixture (often on a piece of paper that is permeated with the indicator). By comparing the color of the indicator or indicator mixture that is in contact with the sample to the colors of the dyes in their acidic and basic forms, it becomes possible to determine the pH of the solution.
Q-2What are conductance?
Conductance is the measure of how easily electricity flows along a certain path through an electrical element, and since electricity is so often explained in terms of opposites, conductance is considered the opposite of resistance. In terms of resistance and conductance, the reciprocal relationship between the two can be expressed through the following equation:
R = 1/G,
G=1/R;
Where,
R = resistance
G = conduction
Q-3Explain spectroscopic techniques.
Spectroscopic techniques have been applied in virtually all technical fields of science and technology. Radio frequency spectroscopy of nuclei in a magnetic field has been employed in a medical technique called Magnetic Resonance Imaging (MRI) to visualize the internal soft tissue of the body with unprecedented resolution. Microwave Spectroscopy was used to discover the so-called three-degree black body radiation, the remnant of the big bang (i.e., the primeval explosion) from which the universe is thought to have originated. The internal structure of the proton and neutron and the state of the early universe up to the first thousandth of a second of its existence are being unraveled with spectroscopic techniques using high-energy particle accelerators. The constituents of distant stars, intergalactic molecules, and even the primordial abundance of the elements before the formation of the first stars can be determined by optical, radio, and X-Ray spectroscopy. Optical spectroscopy is used routinely to identify the chemical composition of matter and to determine its physical structure.
Q-4What is selection rule?
On the irradiation of the sample with infrared electromagnetic radiation then its frequency can be measured by the Infrared spectroscopy. It is a technique used to study the vibrations between atoms because atomic vibrational excitations occur in the infrared region of the electromagnetic spectrum. The bonds between atoms can be thought of as a spring connecting two masses. In the spring-mass analogy the moving system can be approximated by a simple harmonic oscillator. The frequency oscillation is proportional to
√k/m
Where,
k is the spring constant
m is the mass of the object.
Q-5What are monochromator?
Monochromator used to measure the wavelength of the light. Monochromator is placed between the sample and the source of light passing from that the light passes to the detector. Here the monochromatic used to detect the wavelength of the light form the source. While on moving further there is a Double beam Ultraviolet-Visible instrument is used here is a splitter and a series of mirrors to get the beam to a reference sample and the sample to be analyzed, this allows for more accurate readings. In the simultaneous Ultraviolet-Visible instruments monochromator is removed hence it has diode array detectors that allow the instrument to simultaneously detect the absorbance at all wavelengths. This instrument is much faster and accurate than double and single Ultraviolet-Visible instrument.
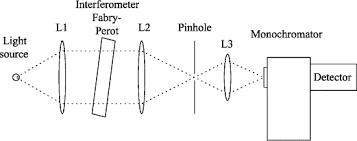
Q-6Explain UV-Visible spectroscopy?
Ultraviolet and Visible Spectroscopy helps ion the determination of the absorbance spectra of the compound in the solution. The absorbance of light energy or the electromagnetic radiations are observed. This is responsible for the exciting the electron from the ground state to the first singlet excited state of a compound. The UV-Visible region of energy for the electromagnetic spectrum covers 1.5 - 6.2 eV which relates to a wavelength range of 800 - 200 nm.
Q-7Explain the applications of UV-Visible Spectroscopy.
1- This is majorly used in the analytical chemistry.
2- This can be used as a detector for the High-performance liquid chromatography.
Ultraviolet-Visible Spectroscopy is used as the semiconductor in the industry to measure the thickness and optical properties of wafer.
Q-8Explain rotational spectroscopy.
Rotational Spectroscopy is also called as the microwave spectroscopy. It is the measurement of energy of transition between quantized rotational states of molecules in the gas phase. The rotational spectra of non-polar molecules cannot be observed by this method while it can be measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. The molecules of the Rotational spectroscopy are classified according to their symmetry. The important feature of molecular rotational spectroscopy for chemical analysis is that the resolution of the spectral transitions is sufficiently high that the spectra of isomers with only small changes in mass distribution can be measured without spectral overlap. For example, the 13C- isotopologues of molecules, isomers created when a single carbon atom is isotopically substituted, are routinely resolved in the instruments used for rotational spectroscopy. The extreme sensitivity changes to in the mass distribution make rotational spectroscopy well-suited to distinguishing diaestromers of molecules with multiple chiral centers. A second advantage of the high-resolution of the spectrometers is that complex sample mixtures can be analyzed directly without spectral overlap. An example of the rotational spectrum from the vapor head space of an essential oil will be presented later to illustrate this capability.
Q-9Enlist the applications of UV-Visible spectroscopy.
1- Rotational Spectroscopy is used in the determination of molecular structure in the gas phase molecule.
2- Rotational Spectroscopy also provide the information about the electronic structures of molecule.
This plays the major role in exploration of chemical composition of interstellar medium.
Q-10Explain infrared spectroscopy.
The change in the vibrational motion of molecule is absorbed in the Infrared Region this is why it is also called as the Infrared Spectroscopy. If vibration in the molecule persists then it should change its dipole moment that may be the magnitude form or oriented with its direction. When taking the example of Polar molecule (A-B) as the polar molecule shows the property of the high electronegative atom attracts the electron pair towards it so that it gets partial negative and partial positive charges. So, in the polar molecule the atoms are making rotation therefore one side is positive pole while other is negative but due to the rotation at its own axis then there is no change in the magnitude effects hence it posses the generation of magnetic field which in result there in the electric flux or there is a change in the direction.
Q-11Enlist the applications of Infrared spectroscopy.
1-Infrared technique is used in organic and inorganic chemistry.
2-This is used in quality control, dynamic measurement.
3-This is majorly used in the forensic labs.
4-Infrared Spectroscopy is used in measuring the degree of polymerization in manufacturing the polymers.
Unit 08
Analytical Techniques
Q-1Explain about the measurement of pH.
The pH of a solution is the negative logarithm (base 10) of the activity of the hydrogen ions (H+) in the solution. In solutions of low ionic strength, pH can be defined as the negative logarithm of the molar concentration of the hydrogen ions because activity and concentration are nearly identical in these solutions. One method for determining pH is by use of a chemical acid-base indicator, which consists of a dye that is either a weak acid or a weak base. The dye has one color in its acidic form and a second color in its basic form. Because different dyes change from the acidic to the basic form at different pH values, it is possible to use a series of dyes to determine the pH of a solution. A small portion of the dye or dye mixture is added to the analyte or a portion of the analyte is added to the dye mixture (often on a piece of paper that is permeated with the indicator). By comparing the color of the indicator or indicator mixture that is in contact with the sample to the colors of the dyes in their acidic and basic forms, it becomes possible to determine the pH of the solution.
Q-2What are conductance?
Conductance is the measure of how easily electricity flows along a certain path through an electrical element, and since electricity is so often explained in terms of opposites, conductance is considered the opposite of resistance. In terms of resistance and conductance, the reciprocal relationship between the two can be expressed through the following equation:
R = 1/G,
G=1/R;
Where,
R = resistance
G = conduction
Q-3Explain spectroscopic techniques.
Spectroscopic techniques have been applied in virtually all technical fields of science and technology. Radio frequency spectroscopy of nuclei in a magnetic field has been employed in a medical technique called Magnetic Resonance Imaging (MRI) to visualize the internal soft tissue of the body with unprecedented resolution. Microwave Spectroscopy was used to discover the so-called three-degree black body radiation, the remnant of the big bang (i.e., the primeval explosion) from which the universe is thought to have originated. The internal structure of the proton and neutron and the state of the early universe up to the first thousandth of a second of its existence are being unraveled with spectroscopic techniques using high-energy particle accelerators. The constituents of distant stars, intergalactic molecules, and even the primordial abundance of the elements before the formation of the first stars can be determined by optical, radio, and X-Ray spectroscopy. Optical spectroscopy is used routinely to identify the chemical composition of matter and to determine its physical structure.
Q-4What is selection rule?
On the irradiation of the sample with infrared electromagnetic radiation then its frequency can be measured by the Infrared spectroscopy. It is a technique used to study the vibrations between atoms because atomic vibrational excitations occur in the infrared region of the electromagnetic spectrum. The bonds between atoms can be thought of as a spring connecting two masses. In the spring-mass analogy the moving system can be approximated by a simple harmonic oscillator. The frequency oscillation is proportional to
√k/m
Where,
k is the spring constant
m is the mass of the object.
Q-5What are monochromator?
Monochromator used to measure the wavelength of the light. Monochromator is placed between the sample and the source of light passing from that the light passes to the detector. Here the monochromatic used to detect the wavelength of the light form the source. While on moving further there is a Double beam Ultraviolet-Visible instrument is used here is a splitter and a series of mirrors to get the beam to a reference sample and the sample to be analyzed, this allows for more accurate readings. In the simultaneous Ultraviolet-Visible instruments monochromator is removed hence it has diode array detectors that allow the instrument to simultaneously detect the absorbance at all wavelengths. This instrument is much faster and accurate than double and single Ultraviolet-Visible instrument.
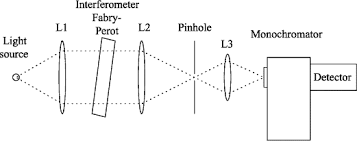
Q-6Explain UV-Visible spectroscopy?
Ultraviolet and Visible Spectroscopy helps ion the determination of the absorbance spectra of the compound in the solution. The absorbance of light energy or the electromagnetic radiations are observed. This is responsible for the exciting the electron from the ground state to the first singlet excited state of a compound. The UV-Visible region of energy for the electromagnetic spectrum covers 1.5 - 6.2 eV which relates to a wavelength range of 800 - 200 nm.
Q-7Explain the applications of UV-Visible Spectroscopy.
1- This is majorly used in the analytical chemistry.
2- This can be used as a detector for the High-performance liquid chromatography.
Ultraviolet-Visible Spectroscopy is used as the semiconductor in the industry to measure the thickness and optical properties of wafer.
Q-8Explain rotational spectroscopy.
Rotational Spectroscopy is also called as the microwave spectroscopy. It is the measurement of energy of transition between quantized rotational states of molecules in the gas phase. The rotational spectra of non-polar molecules cannot be observed by this method while it can be measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. The molecules of the Rotational spectroscopy are classified according to their symmetry. The important feature of molecular rotational spectroscopy for chemical analysis is that the resolution of the spectral transitions is sufficiently high that the spectra of isomers with only small changes in mass distribution can be measured without spectral overlap. For example, the 13C- isotopologues of molecules, isomers created when a single carbon atom is isotopically substituted, are routinely resolved in the instruments used for rotational spectroscopy. The extreme sensitivity changes to in the mass distribution make rotational spectroscopy well-suited to distinguishing diaestromers of molecules with multiple chiral centers. A second advantage of the high-resolution of the spectrometers is that complex sample mixtures can be analyzed directly without spectral overlap. An example of the rotational spectrum from the vapor head space of an essential oil will be presented later to illustrate this capability.
Q-9Enlist the applications of UV-Visible spectroscopy.
1- Rotational Spectroscopy is used in the determination of molecular structure in the gas phase molecule.
2- Rotational Spectroscopy also provide the information about the electronic structures of molecule.
This plays the major role in exploration of chemical composition of interstellar medium.
Q-10Explain infrared spectroscopy.
The change in the vibrational motion of molecule is absorbed in the Infrared Region this is why it is also called as the Infrared Spectroscopy. If vibration in the molecule persists then it should change its dipole moment that may be the magnitude form or oriented with its direction. When taking the example of Polar molecule (A-B) as the polar molecule shows the property of the high electronegative atom attracts the electron pair towards it so that it gets partial negative and partial positive charges. So, in the polar molecule the atoms are making rotation therefore one side is positive pole while other is negative but due to the rotation at its own axis then there is no change in the magnitude effects hence it posses the generation of magnetic field which in result there in the electric flux or there is a change in the direction.
Q-11Enlist the applications of Infrared spectroscopy.
1-Infrared technique is used in organic and inorganic chemistry.
2-This is used in quality control, dynamic measurement.
3-This is majorly used in the forensic labs.
4-Infrared Spectroscopy is used in measuring the degree of polymerization in manufacturing the polymers.