Unit 04
Polymers and Fibers
Question Bank
Q-1What are different type of polymerization?
Types of polymerization:
Polymers can be synthesized by the following polymerization processes:
- Addition polymerization or chain polymerization
- Condensation or step or step growth polymerization
III. Copolymerization
Addition polymerization:
The addition polymerization is the process in which the linking together of monomer molecules by a chain reaction is observed. Polymer synthesized by addition polymerization has the same empirical formula as that of monomer. No molecule is evolved during polymerization and the polymer is an exact multiple of the original monomeric molecule.
Condensation polymerization:
An intermolecular reaction involving two different bifunctional reactants with affinity for each other and taking place through repeated condensation reaction is known as condensation polymerization.
Copolymerization:
Addition polymerization involving a mixture of two or more suitable or compatible monomers gives a copolymer and the process is known as copolymerization. A reaction in which a mixture of two or more monomers is allowed to undergo polymerization is known as copolymerization. The polymer is known as copolymer.
E.g.: Copolymerization of styrene and methyl methacrylate
Q-2Explain free radical polymerization.
The formation of polymer from the successive addition of free-radical building blocks through the polymerization is called as the free radical polymerization. In order to obtain the wide variety of different polymers and material composites the free radical polymerization plays a major role in it. The relatively non-specific nature of free-radical chemical interaction make it one of the most versatile forms of polymerization which allows a facile reaction of polymeric free radical chain ends and other chemical or substrates. The monomers from which addition polymers are made are alkenes. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates, and this is the mechanism followed by most polymerization. The monomers are initiated by traces of oxygen, the pure compounds are stabilized by radical inhibitors.
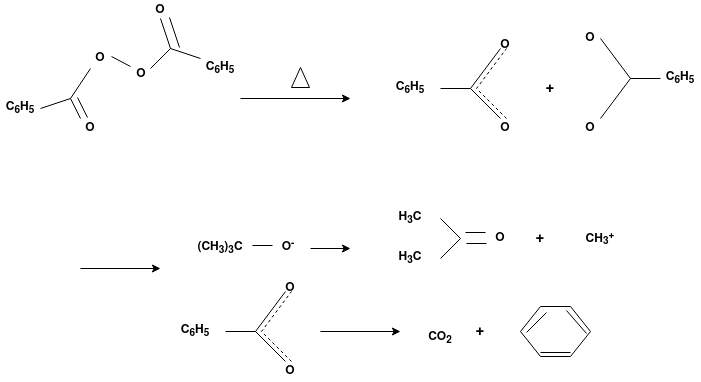
Radical Initiators
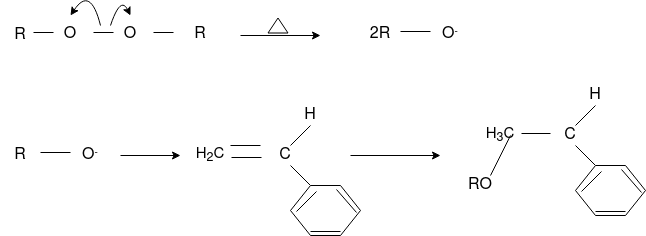
Chain Initiation
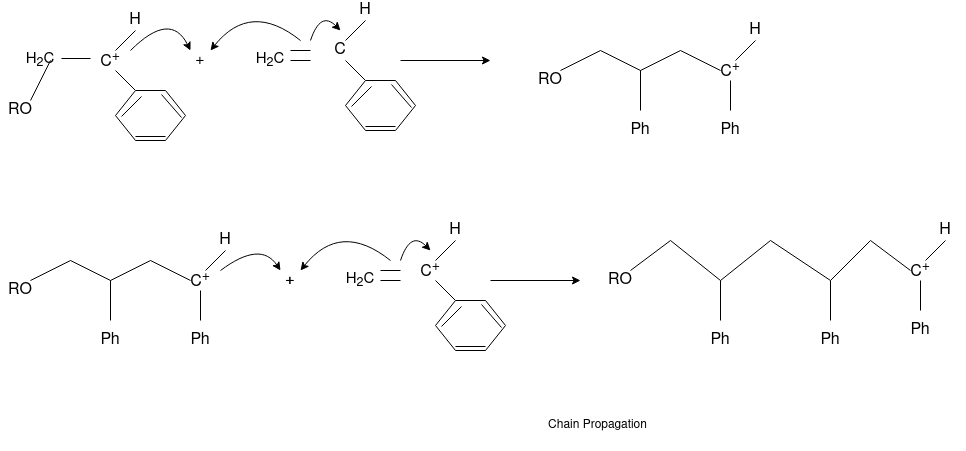
Chain Propagation
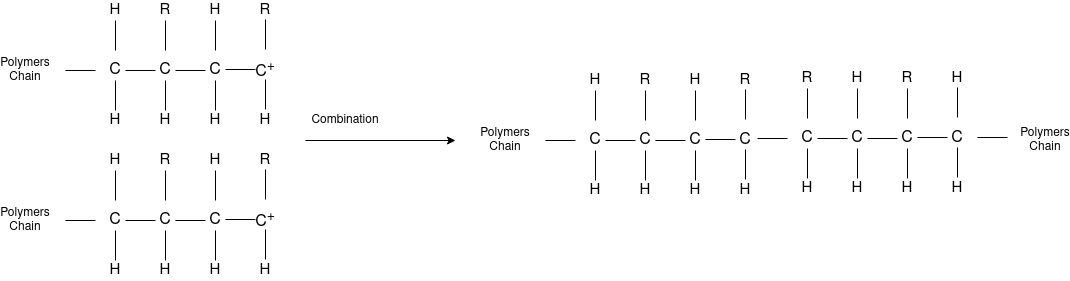
Chain Termination

Chain Transfer Reaction
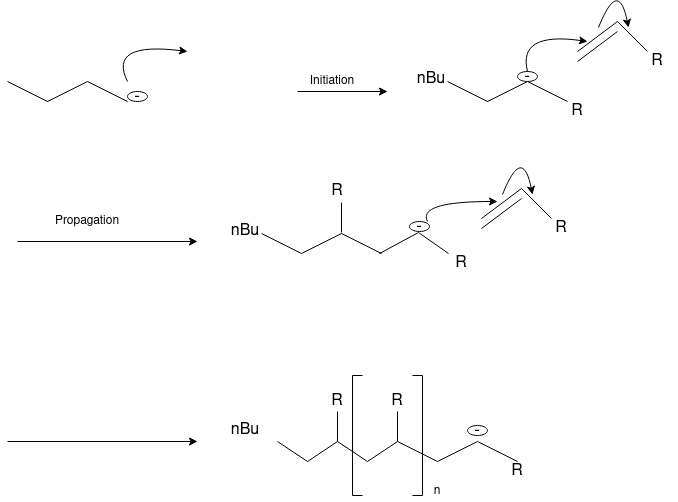
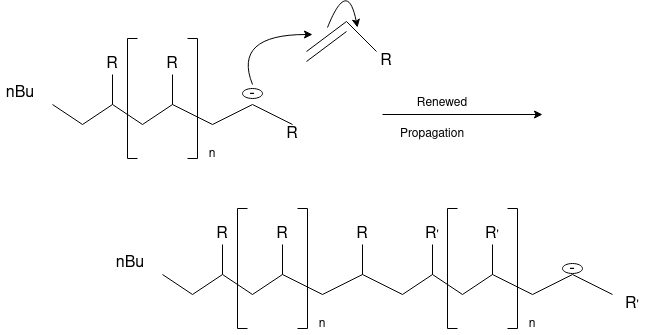
Q-3What is Ionic Polymerization?
Alkenes can polymerize under the influence of both cationic and anionic initiators. As with free radical polymerizations, the ionic processes involve initiation, propagation and (sometimes) termination steps.
Q-4Explain cationic and anionic polymerization.
 Cationic polymerizations are typically initiated by carbocations, generated by protonation of an alkene with a strong acid such as sulfuric or tri-fluoro methane sulfonic acid.
Cationic polymerizations are typically initiated by carbocations, generated by protonation of an alkene with a strong acid such as sulfuric or tri-fluoro methane sulfonic acid.
Anionic Polymerization: Anionic polymerizations are typically initiated by carbanions such as organolithium compounds.
Q-5Explain about the natural rubber.
Natural Rubber is a high molecular weight hydrocarbon polymer represented by the formula (C5H8)x. It is obtained from a milk emulsion called latex by tapping the bark of the tree. “Have a brasiliensis”. It is a polymer of isoprene units.
n H2C = C – CH = CH2 ( H2C – C = C – CH2 )n
CH3 CH3
The polymer chain of natural rubber is made of 2000 to 3000 monomer units.
Processing of Natural Rubber:- By cutting the bark of rubber tree the milky colloidal rubber milk is obtained. The main constituent of rubber latex is 25-45% of rubber and the remaining are water, protein & resinous materials. The rubber latex is coagulated by using 5% acetic acid and made in to sheets. The rubber sheets are cured under mild heat and then subjected to further processing.
Q-6Explain vulcanization of rubber.
Vulcanization is a chemical process that converts natural rubber and other polydiene elastomers into cross-linked polymers. The most common vulcanization agent is sulfur. It forms bridges between individual polymer molecules when heated with rubber. Often a catalyst and initiator are added to accelerate the vulcanization process. The cross-linked elastomers have much improved mechanical properties. In fact, unvulcanized rubber has poor mechanical properties and is not very durable.
- Mixing of crude rubber with about 5-30% of sulfur (cross-linking agent) and other additives such as:
- Activator (commonly zinc oxide or stearic acid),
- Accelerator (guanidines, thiazoles, dithiocarbamates, xanthates, thiurams) ,
- Coagulants (acetic acid, calcium chloride),
- Anti-oxidants (amines, phenolics, phosphites),
- Color pigments,
- Surfacants,
- Softeners,
- Ant-foaming agents,
- Anti-tack agents (Rosin derivates, coumarone-indene resins, aliphatic petroleum resins, alkyl-modified phenol-formaldehyde resins).
Slow cross-linking starts at this stage. It is neccessary to avoid active vulcanization during mixing, which may cause cracks formation at the molding stage.
- Molding (shaping) the rubber mixture. The rubber must be shaped prior to heating stage since cross-linking makes shaping impossible.
Heating the mixture to 250-400ºF (120-200ºC). Increased temperature speeds up the vulcanization process resulting in fast and complete cross-linking. C-S bonds replace C-H bonds linking chain polyisoprene molecules. Each link is formed by one to seven sulfur atoms.
Q-7Enlist the application of rubber.
(i) The major application of natural rubber is in the manufacture of tyres.
(ii) In heavy duty tyres, the major portion of the rubber used is natural rubber.
(iii) The tank linings in chemical plants where corrosive chemicals are stored are prepared from rubber.
(iv) To reduce machine vibrations, rubber is used for sandwiching between two metal surfaces.
(v) Foam rubber is used for making cushions’, matrices, padding etc. toys and sports items are manufactured from natural rubber.
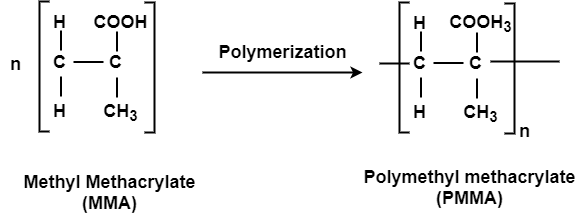
Q-8Explain the preparation and properties of PMMA.
Preparation:
This is an important thermoplastic resin. It is obtained by polymerization of methyl methacrylate which is an ester of methyl acrylic acid, CH2=C(CH3)COOH, in presence of acetyl peroxide or hydrogen peroxide. It is an acrylic polymer.
Properties:
(i) It is hard, fairly rigid material with a high softening point of about 130-14rfC.
(ii) It becomes rubber like at a temperature above 6oC.
(iii) It has outstanding shape-forming properties due to wide span of temperature from its rigid state to viscous.
(iv) It has high optical transparency.
It has high resistance to sunlight and ability of transmission light accurately.
Q-9Discuss about polyethylene and its uses.
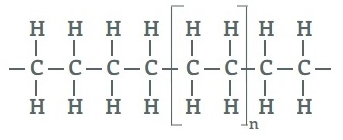
Polyethylene is a lightweight, durable thermoplastic with variable crystalline structure. It is one of the most widely produced plastics in the world. Polyethylene is used in applications ranging for films, tubes, plastic parts, laminates, etc. in several markets (packaging, automotive, electrical, etc.).
Polyethylene is made from the polymerization of ethylene or ethene monomer. Polyethylene chemical formula is (C2H4)n.
Uses:
- Polyethylene is used in packaging products. This plastic is often employed for the production of plastic bags, plastic films, bottles, etc.
- Polyethylene is used in crates, trays, jugs that carry milk or fruit juices.
- High-density polyethylene is used in toys, garbage containers and ice trays.
- HDPE is also used in ropes, fishing nets, agricultural nets, and industrial fabrics.
- Low-density polyethylene (LDPE) is widely used in the production of squeeze bottles, garbage bags, laminations, and food packaging due to its high flexibility and low cost.
- LDPE is also used in pipes and fittings.
Polyethylene is also used for cable jacketing since it is a good insulator of electric current.
Q-10Discuss about the PVC and its uses.
The monomer used for the manufacture of PVC is vinyl chloride.Vinyl chloride is prepared by treating acetylene with HCl at 60-800 c and in presence of a metal oxide catalyst.
Metal oxide
CH + HCl CH2 = CHCl
(Acetylene) 60 – 800 c Vinyl chloride
Poly vinyl chloride is produced by heating vinyl chloride in presence of benzyl peroxide or H2O2.
Benzoyl peroxide
n CH2 = CH ( CH2 – CH )n Cl Polymerisation Cl
Vinyl chlorideat 30 – 800 c PVC
Uses:
- PVC is used in making seals & gaskets, which have to withstand high temperature.
- It is also used for insulation of electrical items and for making non-sticky surface coating, particularly for cooking utensils.
Teflon used as insulating material for motors, transformers, cables, wires, fitting etc.
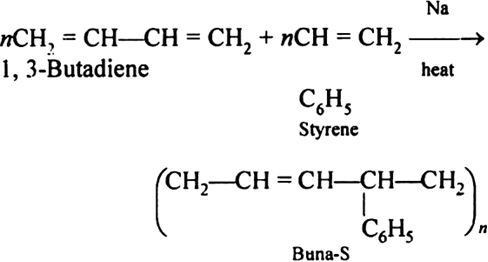
Q-11Explain Buna-S.
Buna-S is also known as the styrene-butadiene. It is a copolymer of butadiene (75%) and styrene (24%). Buna is derived from the Bu-Butadiene while Na is Sodium or Natrium and S is Styrine. Buna-S is the replacement of natural rubber while styrene, 2 monomers and butadiene play a major role in its derivation where as these 2 monomers is polymerized by two basically different process i.e., from solution (S-SBR) or as an emulsion (E-SBR). It is prepared by the copolymerization of butadiene & styrene.
It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide as a catalyst at 5o C and this is the reason why the product is called as cold rubber. The obtained rubber is called as the Styrene Butadiene Rubber (SBR).
Q-12Write about the liquid crystal.
There are three state of matter i.e.; Solid, Liquid, Gas. Liquid Crystal is the fourth state that enters under the right conditions and consequences. The matter which shows the property between the conventional Liquid and Solid Crystals. This can be simplified in this manner that the Liquid Crystals may flow like the liquid but the molecule of the crystal may be in the crystal form. The molecules in the liquid crystal do not exhibit any positional order instead they represent the orientational order. They tend to orient in one direction more than other hence this direction is known as the director of the Liquid Crystals.
There are several phases in Liquid Crystal.
1-Mesomorphic Phases
1.1-Thermotropic Liquid Crystal
1.2-Lyotropic Liquid Crystal
Thermotropic Liquid Crystal class can be prepared by heating. The known crystals are organic compounds. Ex.- bis-(p-methylbenzal)-p.
While at another end the Lyotropic Liquid Crystal are prepared by the mixture of two or more components.
Applications
Liquid Crystal Display:
Polarization: Light wave is an electromagnetic wave in which time barring electric field vector E bar and magnetic field vector V bar are mutually perpendicular to each other and perpendicular to the direction of propagation of light wave. In a light wave electric field vector vibration occurs in different planes such light wave isunpolarized light wave. If it is confined in particular direction then such light wave is polarized light wave. This phenomenon is called Polarization. When unpolarized light pass through the polarizer then the vibration of polarizer tends to polarize the light. While in Liquid Crystal Display the intensity of light can be controlled at a given point by changing the orientation of molecules. The optical properties of the liquid crystal can often be manipulated by subjecting it to a magnetic or electric field that changes the orientation of the molecules. The number of regions or the pixels in there per unit area responsible for the following conditions:
Very low Resolution
Low Resolution
Improved Resolution
High Resolution