Module 6
Stereochemistry
Question Bank
- Define Isomerism and its types.
Two or more compounds having the same molecular formula but different physical and chemical properties are called isomers and the phenomenon is known as isomerism. There are two types of isomerism
- Structural isomerism.
- Stereoisomerism.
2. Give one difference between metamerism and Tautomerism?
The compounds that have same formula but differ in alkyl chains on either side of the functional group are called as metamers and the phenomenon is known as metamerism.
Tautomerism occurs due to the 1,3-migration of a hydrogen atom within the same molecule. The best example of tautomerism is keto-enol tautomerism, where one form contains the keto group (>C=O) and the other form contains the enol (en + ol) group.
3. Differentiate between Enantiomers and Diastereomers?
The key difference between diastereomers and enantiomers is that diastereomers of a molecule are not enantiomers are mirror images of each other mirror images of each other while.
Enantiomers contain chiral centers that are non-superimposable & mirror images. They only come in pairs!
Diastereomers contain chiral centres which are non-superimposable but are NOT mirror images. There can be more than 2 depending on the number of stereocenters.
4. What is optical activity?
The optical activity of a substance, is the tendency of a substance to rotate along the plane of polarization of a beam of light that is passed through it. (In a plane-polarized light, the vibrations of the electric field are confined to a single plane.) The intensity of optical activity of a substance is expressed in terms of a quantity, called specific rotation, Optical activity was first observed in quartz crystals in 1811 by a French physicist, francois Arago. Another French physicist, Baptiste, found in 1815 that liquid solutions of tartaric acid or of sugar are optically active, as are liquid or vaporous turpentine. Louis Pasteur was the first to recognize that optical activity arises from the dissymmetric arrangement of atoms in the crystalline structures or in individual molecules of certain compounds.
5. Explain Conformational Isomerism.
Conformational isomers are those isomers where the relative positions of few atoms differ in the molecule in the three-dimensional space because of the rotation about sigma bonds.
6. Who Introduced Conformational analysis? What are the two possible conformation analysis?
A conformational analysis is a study of the energetics of different spatial arrangements of atoms relative to rotations about bonds. Conformational analyses are assisted greatly by a representation of molecules in a manner different to skeletal structures
In ethane specifically, we can imagine two possible "extreme" conformations. In one case, the dihedral angle is 0° and the hydrogens on the first carbon line up with or eclipse the hydrogens on the second carbon. When the dihedral angle is 0° and the hydrogens line up perfectly, ethane has adopted the eclipsed conformation. The other extreme occurs when the hydrogens on the first carbon are as far away as possible from those on the second carbon; this occurs at a dihedral angle of 60° and is called the staggered conformation
7. Define Specific rotation?
Optical rotation is the rotation of plane-polarized light by a substance. This rotation can be either clockwise or anticlockwise. The compounds that are capable of this rotation are enantiomers. The standard measurement for optical rotation of a specific chemical compound is called specific rotation.
8. Explain Symmetry of a molecule
The symmetry of a molecule describes how its different parts relate to one another geometrically. Symmetry plays an important role in many areas of chemistry, with effects on:
- Physical properties: e.g. Dipole moment, chirality
- Spectroscopic properties: geometric equivalence of groups or nuclei, transition intensities.
- Bonding interactions: the bonds need to overlap of atomic orbitals of correct symmetry.
9. Explain the Isomerism in Transition Metal Ions?
Isomers are different species but have the same chemical formula. Geometric isomers are often formed by the Transition metals, in this case the same atoms are connected through the same types of bonds but show differences in their orientation in space. Isomers are formed with the Coordination complexes having two different ligands in the cis and trans positions from a ligand of interest. For example, the octahedral [Co (NH3)4Cl2] + ion has generally two isomers. In the cis configuration, the ligands of the two chloride ions are adjacent to each other (see the figure below). The other isomer, the trans configuration, has the two chloride ligands directly across from one another.
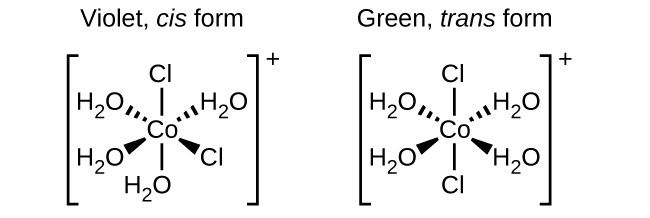
In both cases the spatial arrangement differs in both compound resulting in the components having different properties. The cis and trans isomers of [Co(H2O)4Cl2]+ contain the same ligands attached to the same metal ion.
In case of geometric isomers, different substances have different chemical compounds, even though these substances have same formula they differ with respect to properties. For example, the two isomers of [Co(NH3)4Cl2]NO3 differ with respect to colour; the cis form of isomer is violet, and the trans form of isomer is green in colour. Furthermore, added to this these isomers have different characteristics like dipole moments, solubilities, and reactivities.
As an example, to show how the arrangement in space can bring a change on the molecular properties, let look at the polarity of the two [Co(NH3)4Cl2]NO3 isomers. The bond dipoles and their arrangement in space determine the polarity of a molecule or ion. On observation in one isomer, cis chloride ligands cause more electron density on one side of the molecule compared to the other, making it polar. For the trans isomer, each ligand is directly across from an identical ligand, so the bond dipoles cancel out, and the molecule becomes nonpolar.