Unit-3
Question Bank
Q-What is electrochemical cell?
A- Electrochemical cell is a device that generate electrical energy from chemical reactions. The electrochemical cells which generate an electric current are called voltaic cells and those which generate chemical reactions via electrolysis are called electrolytic cells.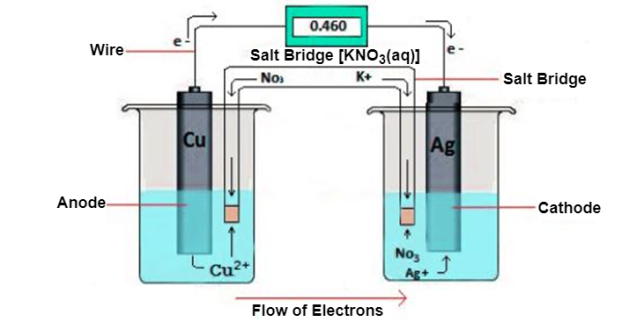
Q-Explain the principle and construction of electrochemical cell.
A- The galvanic cell burns with redox reaction, in this cell oxidation and reduction occurs in separate container called half cell the electron move from of citation half cell to reduction half cell and current flow from reduction half cell to oxides and half cell.
The cell is usually represent as
Zn(s)|Zn2+(aq)||Cu2-|Cu(s)
The two half reaction are
At anode-(oxidition take place)
Zn Zn2+ + 2 e-
At cathode-(reduction take place)
Cu2+ + 2 e- Cu
Net reaction is- Zn +Cu2- Zn2+ +Cu
Construction -

Q-What is electrode Potential?
A- The electrode potential is defined as the Potential difference developed between the metal ions from metal to the solution or from solution to the metal. At equilibrium the potential difference remains constant. The electrode potential of a metal is defined as the direct measure of its tendency to get reduced is called reduction potential, its value is +x volts. Similarly the tendency of an electrode to lose electrons is a measure of its tendency to get oxidized is called oxidation potential.
Q-Explain Calomel Electrode.
A- Calomel electrode is particularly very simple to construct, free from surface sensitivity and accurate to use even in a very normal laboratory.
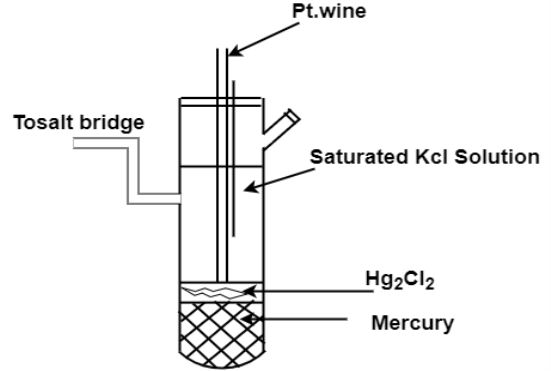
The calomel electrode consists of an inner glass tube and an outer jacket. In the inner glass tube a platinum wire is dipped into mercury which rests on a paste of mercurous chloride, Hg2Cl2 (commercially known as calomel) and mercury. This paste is in contact with KCl present in the outer jacket, through the glass frit plug fixed at the bottom of inner glass tube. The calomel electrode comes in contact with the experimental solution through a frit arranged to the outer jacket. The potential of this electrode depends on the concentration of KCl taken in the outer jacket.
Q-Mention the advantages and limitations of Quinhydrone Electrode.
A- Advantages:
- The quinhydrone electrode is simple to set up and needs no removal of air.
- The reversibility equilibrium is achieved faster than hydrogen gas electrode thereby allowing a quicker measurement.
- PH values of solutions containing reducible substances like Cu2+, Cd2+, unsaturated acids, NO3 - , etc., and catalytic poisons can be measured using quin-hydrone electrode.
Limitations:
- The electrode can not be used at pH values greater than 8.
- This electrode also fails in the presence of strong oxidizing and reducing agents.
Q-Derive the Nernst equation.
A- Gibbs free energy: Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system.
G=H-TS
 Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.
Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.

 G= H- (TS)
G= H- (TS)
While at constant temperature this reaction transform into:


 G= H-T S
G= H-T S
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
E*=E*reduction-E*oxidation………..(i)
 G=-nFE………..(ii)
G=-nFE………..(ii)
Where,
n=no. Of transferred electrons in the reaction
F= Faraday constant
E=Potential Difference.
While when we see in the standard condition then, equation (ii) becomes
 G*=-nFE*………….(iii)
G*=-nFE*………….(iii)
Hence,
Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.

 G= G*+RT lnQ………….(iv)
G= G*+RT lnQ………….(iv)

 Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
−nFE=−nFEo+RTlnQ…………….(v)
On Dividing both sides of the Equation above by −nF,
E=E*−RTnFlnQ(6)……….(vi)
Equation (vi) in the form of log10:
E=E*−2.303RT/nF log10Q…….(vii)
At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation
(vii) can be rewritten:
E=E*−0.0592V/n log10Q……..(viii)

 The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
Then on substituting the these values to Nernst Equation we get,
0=E*-RT/nF In K…….(ix)
At room temperature it becomes;
0=E*-0.0592V/n Log10K
LogK=nE*/0.0592V
Q-Explain Electrochemical Series.
A- The deterioration of materials by chemical process is called as corrosion. In electrochemical corrosion M→M+ + e- is facilitated by the presence of suitable electron acceptor and some time it is also called as depolarizer. Corrosion can also be viewed as the spontaneous return of metals to their ores, the abandoned amount of energy used that were consumed in the mining, refining into useful objects is dissipated by variety of different routes.

Q- What is Potentiometric Titrations?
A- The measurement of suitable indicator electrode with respect to the reference electrode as a function of titrant volume is called as the potentiometric titrations. It provide more accurate data with respect to the data of chemical indicators. These are useful for the detection of the presence of unsuspected species.
Q- What is Lead Storage Battery? Explain its Charge & discharge chemistry.
A-Lead storage battery is the most common device used to store energy in the portable form. This is also called as lead acid battery. Although the batteries are reliable, which contain acidic material inside that required a proper disposal method after its complete use. These batteries have moderate power density and good time. The battery consists of lead grids on its electrodes. The anodic grid opening is filled with spongy lead while the cathodic grid consists of lead oxide (PbO2).
Charge Chemistry of the battery:
Charge batteries are those batteries which can be recharged after single use. In this type of battery each plate contain negative as well as the positive end. The negative plate is of lead while the positive plate is made up of lead oxide in an electrolyte of approx. 4.0M sulphuric acid.
Negative plate reaction:
PbSO4(s) + H+(aq) + 2e– → Pb(s) + HSO4–(aq)
Positive plate reaction:
PbSO4(s) + 2H2O(l) → PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e–
Combining these two reactions, the overall reaction is the reverse of the discharge reaction:
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq)
Discharge Chemistry of the Battery:
The positive and negative plate of the batteries becomes lead sulphate. Due to the loss of sulfuric acid from electrolytes it becomes the water.
Negative plate reaction:
Pb(s) + HSO4–(aq) → PbSO4(s) + H+(aq) + 2e–
Positive plate reaction:
PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e– → PbSO4(s) + 2H2O(l)
Combining these two reactions, one can determine the overall reaction:
Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq) → 2PbSO4(s) + 2H2O(l)
Q- How does corrosion effects economically?
A- To determine the economic cost on the country’s economy there are several studies conducted by different country. Among all the most extensive study was carried out by United State in 1975 and gathered that corrosion cost about $70 billion.
Q-Explain Electrochemical Corrosion.
A- Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by teh elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
Q-What are different types of corrosion? Explain each.
A-
(i) Galvanic Corrosion: Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrodes.
(ii) Pitting Corrosion: This type of corrosion occurs at certain conditions, there is a accelerated corrosion in some areas rather than the uniform corrosion over the substance. This condition includes low level of concentration of oxygen or high concentration of chlorides.
(iii) Microbial Corrosion: Microbial Corrosion is caused by micro-organisms. They commonly referred to as microbiologically influenced corrosion. It applies to both metallic and non-metallic materials with or without oxygen. In the presence of oxygen there are some bacteria that directly oxidize iron to iron oxides and hydroxides while in the absence of oxygen sulphate reducing bacteria are active and produce hydrogen sulphide causes sulphide stress cracking.
(iv) High Temperature Corrosion: The deterioration caused on the metal due to heating. This can cause when the metal is kept in hot atmosphere that too in the presence of oxygen, sulphur, or with any other compound which is capable of oxidizing the material.
(v) Crevice Corrosion: This occurs in confined spaces where access of fluid from the environment is limited such as gaps and contact areas between parts, under gaskets or seals, inside cracks and seams and spaces filled with deposits.
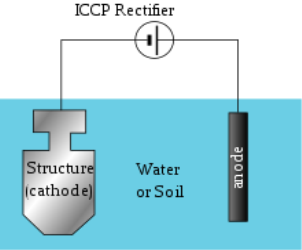
Q-What is Cathodic Protection?
A- This is the technique used to control the corrosion on the surface of metal by formation of cathode layer on an electrochemical cell. There are 2 types of cathodic protections:
(i) Sacrificial Anodic Protection
(ii) Impressed Current Cathodic Protection
Sacrificial Anodic Protection:
The metal surface can be protected from the corrosion by connecting it wire to a more anodic metal. The sacrifice of this more anodic metal to save the metal form corrosion is called as the Sacrificial Anode. The most common metal used for this purpose are Mg, Zn, Al etc.
Applications:
(i) The underground cable and pipeline protection from soil erosion.
(ii) Ships and boat protection from marine corrosion.
(iii) Prevention of rusty water by inserting Mg sheets or rods into domestic water boiler or tanks.
Impressed Current Cathodic Protection:
This is the type of corrosion protection which consist of sacrificial anodes that is connected to an external power source. The external power source is DC power supply, that provides the sufficient current to drive electrochemical reaction required for the cathodic protection to occur.