Unit-2
Question Bank
Q- Mention the different types of water and their hardness levels.
A-
TYPES OF WATER | HARDNESS |
Soft | 0 – 75 |
Moderately hard | 75 – 150 |
Hard | 150 – 300 |
Very hard | Above 300 |
Q-What are different types of hardness? Explain.
A-
- Temporary hardness ( carbonate) :-
- When water containing calcium and magnesium bicarbonates is heated, bicarbonate decompose and from insoluble carbonate and form hydroxide.
- On filtering such water, soft water is obtained.
- The hardness which can be removed by more boiling is referred as ‘temporary hardness or bicarbonate hardness.

 Ca
Ca

Mg 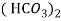
 Mg
Mg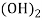 + 2 CO
+ 2 CO 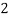
(Bicarbonates)
II. Permanent hardness :-
- The term permanent hardness or non-carbonate is the term applied to the hardness caused by dissolved chlorides, nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
III. Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate, calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
4. As the sulphate and chloride are neutral salts, the hardness caused by presence of calcium and magnesium sulphate, chlorides and nitrates is termed as non-alkaline hardness or non-carbonate hardness.
IV. Total hardness :-
The hardness due to all hardness causing salts, known as total hardness.
Total hardness = temporary + permanent
Q- Explain complexometric method for estimation of hardness.
A- Complexometric titration is a form of volumetric analysis in which the formation of a colored complex is used to indicate the end point of a titration. Complexometric titrations are particularly useful for the determination of a mixture of different metal ions in solution. An indicator capable of producing an unambiguous color change is used to detect the end-point of the titration. Complexometric titration are those reactions where a simple ion is transformed into a complex ion and the equivalence point is determined by using metal indicators or electrometrically.
- The reaction reaches equilibrium rapidly after each portion of titration is added.
- Interfering situations do not arise.
- A complexometric indicator capable of locating equivalence point with fair accuracy is available.
Q-What is Potable Water?
A- “Potable water” simply means water that is safe to drink, and it is becoming scarcer in the world. Increasing use is stressing freshwater resources worldwide, and a seemingly endless list of contaminants can turn once potable water into a health hazard or simply make it unacceptable aesthetically.
Q-What is Coagulation & Flocculation?
A-Water is contaminated with several type of impurities, dirt with other dissolved impurities. Chemicals with the positive charged ions are added to the water to neutralize the negative charges of the dirts, impurities etc. When this occurs, the particles bind with the chemical and form larger particles known as floc.
Q-What is disinfection?
A-After the filtration the disinfectant is added in order to kill any remaining parasites, bacteria, viruses and to protect the water from germs when it is piped to houses.
Q-Explain Ozonization.
Ozone consist of three oxygen atoms that is unstable gas, this ozone gas converted into oxygen by loss of its 1 oxygen atom the free radical formed is highly reactive and very short lived. Ozone is colorless. Ozone has greater disinfection effectiveness against bacteria and viruses with comparison to the chlorination.
Ozone Process
The formation of oxygen into ozone occurs with the use of energy. This process is carried out by an electric discharge field as in the CD-type ozone generators, by ultraviolet radiation as in UV-type ozone generators. In addition to these commercial methods, ozone may also be made through electrolytic and chemical reactions. In general, an ozonation system includes passing dry, clean air through a high voltage electric discharge, i.e., corona discharge, which creates and ozone concentration of approximately 1%. In treating small quantities of waste, the UV ozonation is the most common while large-scale systems use either corona discharge or other bulk ozone-producing methods. Ozoner test strips a must.
The raw water is then passed through a venturi throat which creates a vacuum and pulls the ozone gas into the water or the air is then bubbled up through the water being treated. Since the ozone will react with metals to create insoluble metal oxides, post filtration is required.

Q-What is scale and sludge?
Scale & Sludge Formation: The water evaporates continuously and the dissolved salts concentration increases progressively. When their concentrations reach at saturation point, they are thrown out of water in precipitate form which get stick in inner walls of boiler. If the precipitation takes place in the form of loose or slimy precipitate it is called sludge. While if the precipitated matter forms a hard adhering coating on inner walls of boiler, then it is called as scale. Eg- MgCO3, MgCl2, MgSO4 etc.
Q-Explain Caustic Embrittlement.
The use of high alkaline water in the boiler cause rust in the boiler which is called as Caustic Embrittelment. The presence of sodium carbonate plays a major role during the softening process.
Na2CO3 + H2O → NaOH + CO2
the caustic embrittelment is caused by using sodium phosphate as a softening agent instead of sodium carbonate
Q-Explain Coagulation & Chemical Precipitation.
After all the large objects are removed from the original water source, various chemicals are added to a reaction tank to remove the bulk suspended solids and other various contaminants. This process starts off with an assortment of mixing reactors, typically one or two reactors that add specific chemicals to take out all the finer particles in the water by combining them into heavier particles that settle out. The most widely used coagulates are aluminum-based such as polyaluminum chloride.
Q-Explain Filtration & Ultra Filtration
The next step is generally running through some type of filtration to remove any suspended particles such as sediment, turbidity, and certain types of organic matter. It is often useful to do this early on in the process, as the removal of suspended solids upstream can help protect membranes and ion exchange resins from fouling later on in the pretreatment process. Depending on the type of filtration used, suspended particles can be removed down to under one micron.
Q-What is Deaeration?
At this point in the boiler feed water treatment process, any condensate being returned to the system will mix with the treated makeup water and enter the deaeration or degasification process. Any amount of gasses such as oxygen and carbon dioxide can be extremely corrosive to boiler equipment and piping when they attach to them, forming oxides and causing rust. Therefore, removing these gases to acceptable levels (nearly 100%) can be imperative to the service life and safety of the boiler system. There are several types of deaeration devices that come in a range of configurations depending on the manufacturer, but generally, you might use a tray- or spray-type deaerator for degasification or oxygen scavengers.
Q- What is Calgon Conditioning?
This is the internal water softening method. When Sodium meta Phosphate reacts with boiler water then it prevents the formation of scales & sludges. Calogon converts the scale forming impurities like CaSO4 into soluble complex which are less dangerous to boilers.
 Na2[Na4(PO3)6] 2Na+ + [Na4(PO3)6]2-
Na2[Na4(PO3)6] 2Na+ + [Na4(PO3)6]2-
(Calgon)
 2CaSO4 + [Na4(PO3)6]2- [Ca2(PO3)6]2- + 2Na2SO4
2CaSO4 + [Na4(PO3)6]2- [Ca2(PO3)6]2- + 2Na2SO4
(Soluble Complex
Ion)
On addition of (0.5 to 5 ppm ) prevents the sludge and scale formation.
At low temperature and pressure it forms quite stable & soluble complex with calcium salts.
At high temperature & pressure calogen is converted into sodium orthophosphate which react with calcium salts to form calcium orthophosphates.
 2Na2[Na4(PO3)6] + 6H2O 6Na2P2O7 + 6H2
2Na2[Na4(PO3)6] + 6H2O 6Na2P2O7 + 6H2
 I + CaSO4CaP2O7 + Na2SO4
I + CaSO4CaP2O7 + Na2SO4
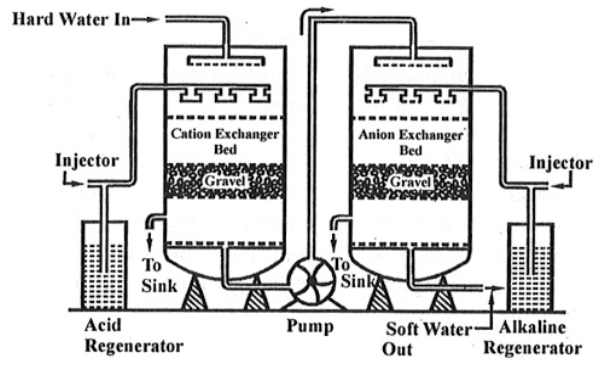
Q-Explain Ion-Exchange Process.
Ion exchange technology is a proven method of producing high purity softened and demineralized water. It is used in most industries that require high purity water and to reclaim water from processes. The Ion exchange process involves the exchanging of contaminant ions for Na+ ions in a softening application and H+ and OH- ions in pure water application. Cations and anions can be removed by the cation and anion exchange resins. Resins containing –COOH, SO3H are capable for exchanging their H+ ions to cationic portion of minerals then it is called as cation exchanger while the resins containing –NH2, NHCH3 are capable for exchanging the anionic portion of the minerals then it is termed as anionic exchanger.
Q-Explain Desalination.
The process of converting up of saline water into fresh water by removing of salts and minerals from the target source is called as the Desalination. Saltwater is desalinated to produce water suitable for human consumption or irrigation. The by-product of the desalination process is brine. Desalination is used on many seagoing ships and submarines. Most of the modern interest in desalination is focused on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water sources.