Question Bank
Unit 05
Q-1 Explain structural isomerism.
Answer: Structural Isomerism:
It is due to the difference in ways in which different atoms or groups are linked to one another within the molecule. In this type of isomerism the isomers have different structure without taking into consideration the arrangement of groups in space. These are further classified as:
Chain Isomerism:
It arises due to the different arrangement of C atoms that is straight or branch chain of C atoms. E.g.: n-butane &iso-butane have same molecular formula C4H10.
n-butane-straight chain
Iso-butane-branched chain
Position Isomerism:
It is due to the difference in position taken up by substituent atoms or groups in same C chain or due to different position of double or triple bond. E.g.: C3H7Cl shows 2 isomeric compound i.e.; n-propyl chloride and iso-propyl chloride.
Ring Chain Isomerism:
This type of isomerism arises due to the difference in type of linking of C-atom & isomer may have either open chain or closed chain structure.
Functional Isomerism:
It arises due to the difference in nature of functional group present in isomeric compound. E.g.: C2H6O
CH3-O-CH3CH3-CH2-OH
Dimethyl ether
Q-2 What are enantiomers?
Answer: Lewis Pastues while studying crystallography of salt of tartaric acid. He observed that optical inactive Na, NH3, tartrate exists as mixture of 2 different type of crystals which mirror images of each other. These crystal which gives the mirror images of each other are called enantiomorphs& the phenomena are called as the enantiomorphism. Although the original mixture was optically inactive each type of crystal when dissolved in water were found to be optically active. The specific rotation of 2 solution were exactly equal but of opposite sign i.e.; 1 solution rotated the PPL to the right or clockwise while other to the left or anti clockwise to the same extent, 2 type of crystal or solution were identical in all other physical and chemical properties. Isomers which are non super imposable mirror images of each other are called enantiomers.
According to the Le-Bell and Van’t Hoff the 4 valencies of C atom are directed towards the 4 corners of a regular tetrahedron at the centre of which C atom lies. Consider a compound CLMNO in which L,M,N,O are 4 different group or atom attached to the C atom may be represented by 2 models:

It is important to know that these 2 molecules can’t be super imposed on each other.
E.g.; Lactic acid and secondary butyl chloride exists as 2 optically active isomers which are enantiomers i.e.; mirror images of each other. Mirror images of 2 compounds are shown as:
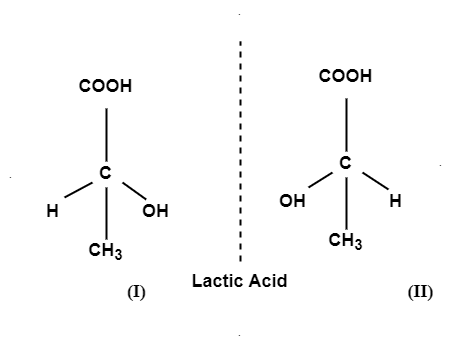
Q-3 what are diastereomers?
Answer: Diastereomers are defined as compounds which have the same molecular formula and sequence of bonded elements but which are non super-imposable, non-mirror images.
Enantiomers and diastereomers commonly called stereoisomer’s fall under the broader concept of isomerism which always involves the comparison of at least two species.Stereoisomers are molecules of identical constitution but nevertheless different. Differences between diastereomers can be expressed in scalar terms, that is by differences in the distances of certain characteristic pairs of atoms. Compounds having identical structure but difference in their configuration i.e.; relative arrangement of atoms or groups within the molecule or different in space are called stereoisomers. The phenomena is called as the stereoisomerism. These compounds are said to have different configuration.
Q-4 Explain Optical Activity.
Answer: Certain substance have strange behavior. When a PPL is passed through the solution of such substance the light coming out of the solution is found to be on different plane. The PPL is rotated. The angle of rotation of PPL is known as the optical rotation. The substance which rotate the PPL to clockwise direction or right direction are dexo-rotatory while the vice versa is called as the levo rotatory. Substance which do not rotate the PPL are said to be optically inactive.
The instrument used for measuring optical rotation is called polarimeter. It consists of light source to nicol prism and in between a tube to hold solution of organic substance. The schematic representation of polarimeter is given as –

Q-5 What do you understand by R-S conformation?
Answer: R means Rectus while the S stands for the Sinister in regular term Rectus is orientation of molecule in clock wise direction while Sinister means the orientation of the molecule in anti clockwise direction. R-S configuration is used to give the identity to the elements attached to the chiral atoms.
The molecule must be arranged according to the priority order.
Priority order depends on the atomic number of the molecule. (the greater atomic number the greater is priority order and vice versa)

Q-6 Explain substitution reaction.
Answer: When a reactant A-B reacts; C comes out as the leaving group i.e.; it has taken a place of B or in other words it has substitution reaction. In other words a substitution reaction is a part of one molecular replaced by other atom or group without causing a change a change in rest of the molecule.
E.g.
A-B+CA-C+B
CH4+ Cl2CH3Cl+ HCl
CH3Cl+Cl2CH2Cl2+HCl
CH2Cl2+Cl2CHCl3+HCl
CHCl3+Cl2CCl4+HCl
The substitution reaction may be brought by free radical nucleophilic or electrophilic reagent.
Mechanism:
The substitution reaction may be completed through following steps:
Chain Initiation:
The formation of chlorine free radical took place by the presence of sunlight or ultraviolet rays.
Cl:ClClo+Clo
Chain Propagation:
The Cl2 free radical replaces H2 atom forming an other free radical which can later again formed Cl2 free radical & thus this chain goes on till all the H2 atoms are reduced by the chlorine.
Clo+H:CH3HCl+oCH3
oCH3+Cl:ClCH3Cl+oCl
oCl+H:CH2ClHCl+oCH2Cl
oCH2Cl+Cl:ClCH2Cl2+oCl
oCl+H:CH2Cl2HCl+oCHCL2
oCHCL2+Cl:ClCHCl3+oCl
oCl+H:CCl3HCl+oCCl3
oCCl3+Cl:ClCCl4+oCl
Chain Termination:
When all hydrogen atom are replaced by Cl than finally 2Cl free radical combines & chains terminates to form Cl molecule.
Clo+oClCl:Cl
Q-7 Explain elimination reaction.
Answer: Elimination Reaction:
Elimination reaction is the type of reaction that is mainly used to convert saturated compounds to the unsaturated compounds that is organic compounds which contain single carbon bonds to the compound which features double or triple carbon bonds. It is a chemical reaction where several atoms either pair or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or action of metals. It can also happen through the process of heating at high temperatures.
Mechanism
The elimination reaction consists of three fundamental eventsthey are:
- C-C pi bond is formed.
- Proton removal.
- There is a breakage in the bond of the leaving group.
Depending on the reaction kinetics, elimination reactions can occur mostly by two mechanisms namely E1 or E2 where E is referred to as elimination and the number represent the molecularity.
E1 Reaction
- This is also called as unimolecular elimination reaction, thereare usually two steps involved – ionization and deprotonation.
- During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
- This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
- In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
- It exhibits first-order kinetics.

E2 Reaction:
- In an E2 mechanism which refers to bimolecularelimination is basically a one-step mechanism.
- Here, the carbon-hydrogen and carbon-halogen bonds mostly break off to form a new double bond.
- However, in the E2 mechanism, a base is part of the rate-determining step and it has a huge influence on the mechanism.
- The reaction rate is mostly proportional to the concentrations of both the eliminating agent and the substrate.
- It exhibits second-orderkinetics.
The E2 mechanism can generally be represented as below. In the below-mentioned representation, B stands for base and X stands for the halogen
Q-8 Explain the synthesis of Aspirin.
Answer: The chemical name for the Aspirin is Acetylsalicyclic acid. It is used as pain killer and fever reducer. Salicyclic acid derive from the willow family of plants which were widely used for treating headache. For the preparation of aspirin the salicyclic acid is reacted with the excess acetic anhydride. Phospohoric acid is used for boosting the procedure of reaction. The excess acetic acid will be quenched with the addition of water. The aspirin product is not very soluble in water so the aspirin product will precipitate when water is added. The synthesis reaction of aspirin is shown below.
Since acetic acid is very soluble in water, it is easily separated from the aspirin product. The aspirin isolated in this step is the “crude product”. A “purified product” can be obtained through re-crystallization of the crude product in hot ethanol. In this experiment, the crude product will be the desired product. The percent yield of the crude product will be determined for this reaction. The purity of the product will also be analyzed. The product will be analyzed by three different methods: melting point, titration, and spectroscopic assay. C and the melting point range of the salicylic acid. The melting point range of pure aspirin is 138-140 C. If impurities are present in your crude sample, the melting point rangestarting material is 158-161 for your product will be lower than the range of pure aspirin. Also, your melting point range may be greater than 2 degrees.
Q-9 Explain the synthesis of Paracetamol from nitro phenol.
