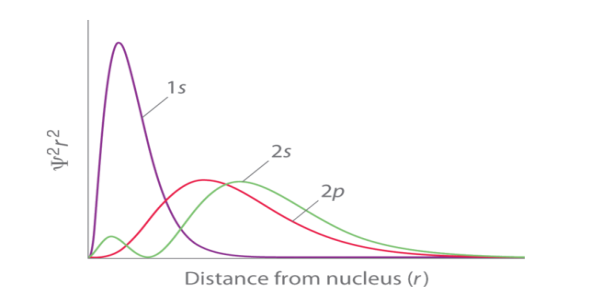
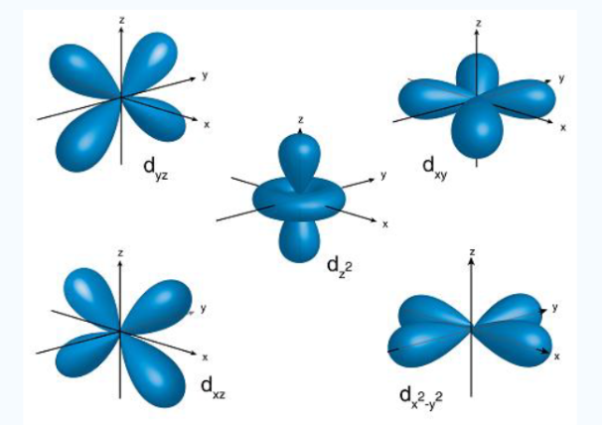
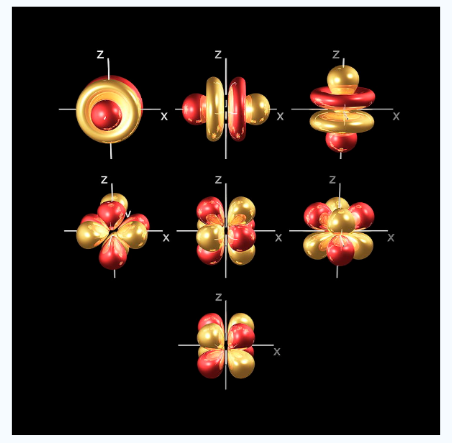
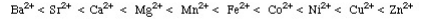Electrons of higher can possess different electrons of lower energy between the electron and the nucleus, thereby lowering the positive charge experienced by the high energy electron.The shielding effect is named as it gives a balance between the repulsion between valence and inner electrons the attraction of valence electrons. The shielding effect also explains the trend in atomic size followed in the periodic table and also explains why valence electrons are readily removed from an atom Q2) Write a short on Penetration of orbitals? It is the ability of an orbital to attract an electron. This process is achieved together with release of energy. The high extent of penetration isa feature of orbitals. The penetration effect of S orbital is the maximum because of the closeness to the nucleus that are the p, d and f orbitals. It describes the proximity to which an electron can approach to the nucleus. In a multi electron system electron penetration is defined by an electron’s relative electron density near the nucleus of an atom. Electrons in different orbitals have different electron densities around the nucleus.
Fig. 1: Orbital Penetration. |
|
|
|
|
|
Where l = 0, 1, 2, 3 for s, p, d, f orbitals respectively.
|
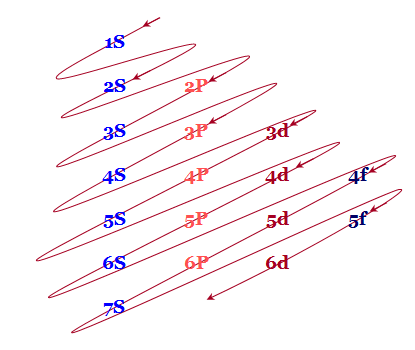
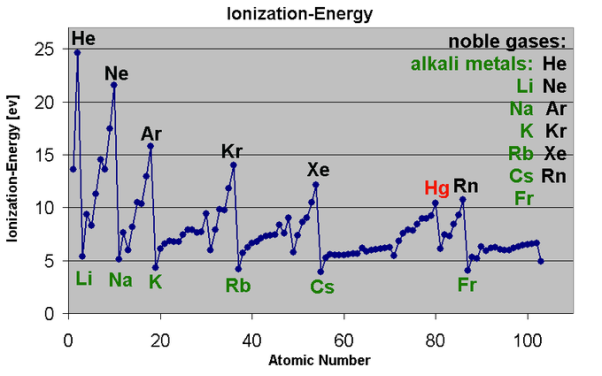
1S < 2S < 2P < 3S < 3P < 4S < 3d < 4P < 5S < 4d < 5P < 6S < 4f < 5d < 6P < 7S < 5f…Q5) What do you understand by Atomic and ionic sizes?A5) Atomic radii or size can be defined in the most simplified manner as the radii (or half the "width") of the spherical atoms. Nonbonding atoms have a much larger, more indefinite or "fuzzy" radius, therefore when atomic radius is described as a periodic trend, the thing that comes to mind is bonding atomic radius. These are the radii of atoms that are chemically bonded to each another. So, if the bond between two Cl atomsin Cl2 is 1.99 angstroms, resulting in chlorine's bonding atomic radius as about 0.99 angstromThese values are then used to estimate bond lengths between different elements in molecules.Atomic radius generally increases from top to bottom down a group. This generally occurs dueto the increase in the principal quantum number, (n). Therefore when we go further down a column, the outer electrons move much further away from the atom, making the atom thus larger.Generally the Atomic radius decreases as we move from left to right across a period. This occurs mainly due to effective nuclear charge. The greater the effective nuclear charge, the more will be the attraction felt by the outer electrons from the nucleus, and the closer the outer electronsare to the nucleus, making the atom smaller. Ionic Size Ionic radii are the radii of ions of elements. These distances are based on distances that exist between ions in ionic compounds.Cations, or positively charged ions, are smaller in size than their "parent" atoms. This is because cations are formed when the outermost orbitals become empty due to absence of electrons. This also decreases electron-electron repulsions. Therefore, the resulting ions are smaller as there are not as many orbitals that are occupied and the effective nuclear charge affecting the remaining electrons increases, pulling electrons in more closely. Anions, or negatively charged ions, are larger in size than their "parent" atoms. This is because of the addition of electrons form these ions, increasing electron-electron repulsions, making the electrons spread out more effectively. Also, effective nuclear charge felt by the outermost electrons decreases. For ions that carry the same charge (i.e. from parent elements of the same group), size increases from top to bottom as we go down a group. Q6) Short note on Ionization energies? The ionization energy of a chemical category (i.e., an atom or molecule) is the energy required to remove electrons from gaseous ions or atoms. This property is also referred to as the ionization potential and is measured in volts. In chemistry, it often refers to one mole of a substance (molar ionization energy or enthalpy) and is reported in kJ/mol. In atomic physics, the ionization energy is typically measured in the unit electron volt (eV). Atoms or molecules which are larger have lower ionization energy, while smaller molecules or atoms tend to have higher ionization energies.The ionization energy is not the same for electrons of different molecular or ionic orbitals. More specifically, the nth ionization energy is the energy required to eliminate the nth electron after the first n-1 electrons are removed. It is perhaps a measure of the tendency of an ion or atom to surrender an electron or the strength of the electron binding. The greater the ionization energy, the more difficult to remove an electron. The ionization energy may be an indicator to show the reaction of an element. Elements with a lower ionization energy tend to be reducing agents and form cations, and thereby combine with anions to form salts.
|
Oxidation states, Hybridization and molecular geometries
Electron affinity reflects the ability of an atom to accept an electron. It is the energy change which occurs when an electron is added to a gaseous atom. Atoms with strong effective nuclear charge have greater electron affinity. The reaction that occurs when an atom takes an electron may be represented as: X + e− → X− + energy Another way to define electron affinity is as the amount of energy needed to remove an electron from a singly charged negative ion: X− → X + e− Key Takeaways: Electron Affinity Definition and Trend
occurs spontaneously.
Electron Affinity Trend Electron affinity is one of the trends that can be predicted using the organization of elements in the periodic table.
Non-metals typically have higher electron affinity values than metals. The element Chlorine strongly attracts electrons whilemercury is the element with atoms that most weakly attract an electron. Electron affinity is more difficult to analyse in molecules because their electronic structure is very complicated. Electron negativity Electron negativity is the property of an atom which increases with its tendency to attract the electrons of a bond. when two bonded atoms that have similar electron negativity values as each other, they share electrons equally in a covalent bond. Usually, the electrons present in a chemical bond are more attracted to one atom (the more electronegative one) than to the other one. This results in a polar covalent bond. If the electron negativity values are very different, the electrons do not show any sharing. One atom essentially takes the bond electrons from the other atom, forming an ionic bond. |
|
Electron negativity is a property of an atom present within a molecule, rather than an fundamental property of an atom by itself. Thus, electron negativity varies depending on an atom's environment or surroundings. However, most of the time an atom displays similar behaviour in different situations. Factors that affect electronegativity include the nuclear charge and the position and number of electrons in an atom. Electron negativity Example The chlorine atom has a higher electron negativity than the hydrogen atom, so the bonding electrons will be closer to the Cl than to the H in HCl molecule. In the O2 molecule, both atoms have the same electron negativity. The electrons in the covalent bond are shared equally between the two oxygen atoms. |

 The vanadium is now in an oxidation state of +2. Further removal of another electron gives the V3+ ion:
The vanadium is now in an oxidation state of +2. Further removal of another electron gives the V3+ ion:
 The vanadium now has an oxidation state of +3. Subsequently the removal of another electron gives a more unusual form of ion, VO2+.
The vanadium now has an oxidation state of +3. Subsequently the removal of another electron gives a more unusual form of ion, VO2+.
 The vanadium at present is in an oxidation state of +4. therefore the oxidation state is not counting the charge on the ion (which was true for the first two cases but not for this particular one).The positive oxidation state is counting the total number of electrons which should have been removed - starting from the element.In the next step it is also possible to remove a fifth electron to give another ion (this may be confused with the one before!). The oxidation state of the vanadium is now +5.
The vanadium at present is in an oxidation state of +4. therefore the oxidation state is not counting the charge on the ion (which was true for the first two cases but not for this particular one).The positive oxidation state is counting the total number of electrons which should have been removed - starting from the element.In the next step it is also possible to remove a fifth electron to give another ion (this may be confused with the one before!). The oxidation state of the vanadium is now +5.
 Every time you oxidise the vanadium by removing another electron from it, its oxidation state increases by 1. if eventually electrons are added again the oxidation state will fall. And finally get back to the element vanadium where the oxidation state of zero.What would be the case if we kept on adding electrons to the element? This cannot be done with vanadium, on the other hand with an element like sulphur.
Every time you oxidise the vanadium by removing another electron from it, its oxidation state increases by 1. if eventually electrons are added again the oxidation state will fall. And finally get back to the element vanadium where the oxidation state of zero.What would be the case if we kept on adding electrons to the element? This cannot be done with vanadium, on the other hand with an element like sulphur.
 According to this equation The sulphur compound has an oxidation state of -2.SummaryOxidation state shows the total number of electrons which have been added from an element (a negative oxidation state) or removed to an element (a positive oxidation state) to get to its present state.Oxidation involves an increase in oxidation stateReduction involves a decrease in oxidation stateThis is a simple pattern and is the single most important thing about the concept of oxidation states. If the oxidation state of an element changes is known during a reaction, instantly it can be predicted whether it is being oxidised or reduced without having to work in terms of electron-half-equations and electron transfers.Working out oxidation states Oxidation states is not calculated by counting the numbers of electrons transferred. It would be a very long process. Instead a few simple rules can be followed, and do some very simple sums!
According to this equation The sulphur compound has an oxidation state of -2.SummaryOxidation state shows the total number of electrons which have been added from an element (a negative oxidation state) or removed to an element (a positive oxidation state) to get to its present state.Oxidation involves an increase in oxidation stateReduction involves a decrease in oxidation stateThis is a simple pattern and is the single most important thing about the concept of oxidation states. If the oxidation state of an element changes is known during a reaction, instantly it can be predicted whether it is being oxidised or reduced without having to work in terms of electron-half-equations and electron transfers.Working out oxidation states Oxidation states is not calculated by counting the numbers of electrons transferred. It would be a very long process. Instead a few simple rules can be followed, and do some very simple sums!Group 2 metals | always +2 |
|
Oxygen | usually -2 | except in peroxides and F2O (see below) |
Hydrogen | usually +1 | except in metal hydrides where it is -1 (see below) |
Fluorine | always -1 |
|
Chlorine | usually -1 | except in compounds with O or F (see below) |
HCl | H2O→ | H+(aq) | + | Cl-(aq) |
NaOH | H2O→ |
| Na+(aq) | + | OH-(aq) |
H+(aq) | + | OH-(aq) | → | H2O |
Acid |
| Base |
| Water |
| Salt |
HCl | + | NaOH | → | H2O | + | NaCl |
HBr | + | KOH | → | H2O | + | KBr |
Acid |
| Base |
|
|
| Salt |
HCl | + | NaHCO3 | → | H2CO3 | + | NaCl |
pH = -log [H+] |
|
|