Unit 01
Atomic and molecular structure
Q-1 Explain Homonuclear Diatomic molecules.
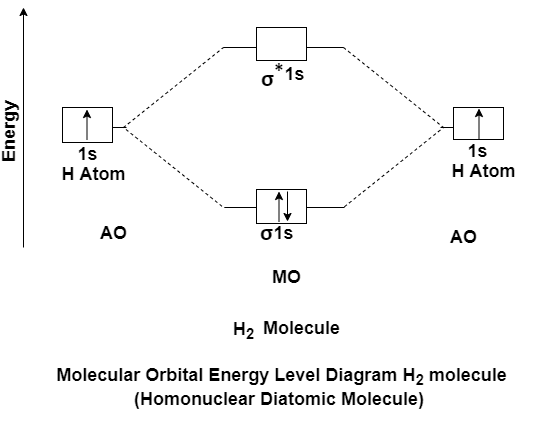
Answer: MO Energy level Diagram for Homonuclear Diatomic molecules:
H2 molecule
H2 molecule consist of two H atoms and their two electrons. Two 1s orbitals give two MOs- one bonding that is σ and another one is antibonding that is σ*. The bonding orbital is in lower in energy state, the two electron occupies the bonding MO. The MO electron configuration of H2 molecule is written as (σ1s) 2.
Energy evolved =
[No. of electron in BMO (-) + No. of electron in Anti Bonding Molecular Orbital * (+)]
=2*(- ) + 0*(+ )
= -2
This evolved energy is called as stabilization energy.
Q-2 Explain Heteronuclear diatomic molecules.
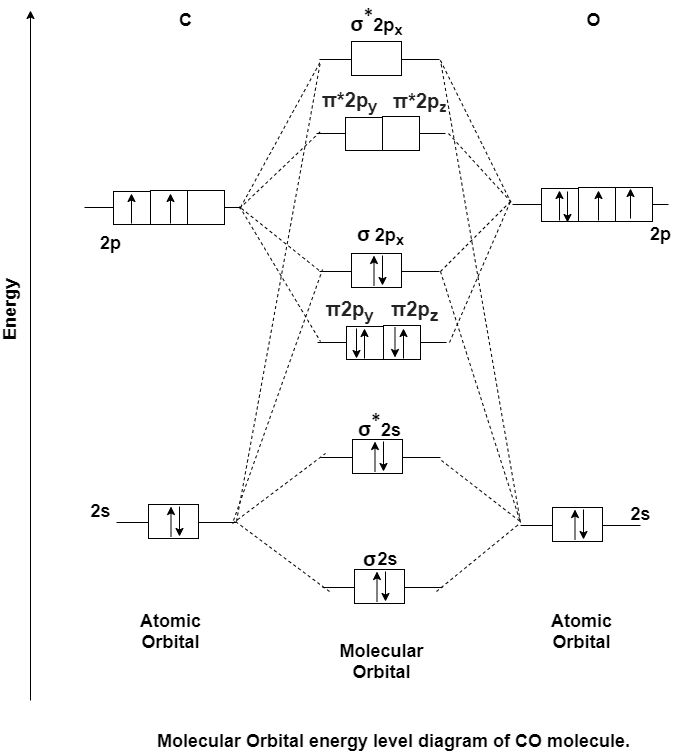
Answer: Carbon Monoxide Molecule (CO)
CO is the hetronuclear diatomic molecule. This molecule is formed by the combination of carbon and oxygen atom. Electronic configuration of carbon atom is 1s2, 2s2, 2p2 and that of oxygen is 1s2, 2s2, 2p4
Stabilisation Energy =
[No. of electron in BMO * (-) + No. of electrons in anti bonding molecular orbital * (+)]
=(8) * (-) + (2) * (+)
= -6
Q-3 Brief about LCAO.
Answer: The approximate method used to represent molecular orbital is called as the Linear Combination of Atomic Orbital. It is a quantum superposition of atomic orbital and a technique for calculating molecular orbital in quantum chemistry.
Rules for the Linear Combination of Atomic Orbital are:-
The combining atomic orbital must be of equal energy or approximately same energy.
If the electron cloud of these two atoms overlaps, then we can obtain the wave function for the molecule by a linear combination of the atomic orbitals ΨA and ΨB. The below equation forms two molecular orbitals.
ΨMO = ΨA + ΨB
Q-4 Explain Pi molecular orbital of butadiene.
Answer: π molecular orbital of butadiene:
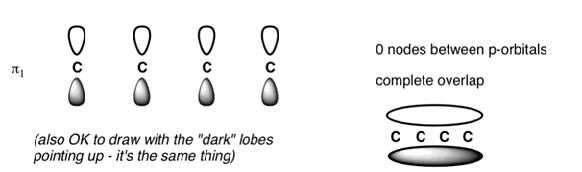
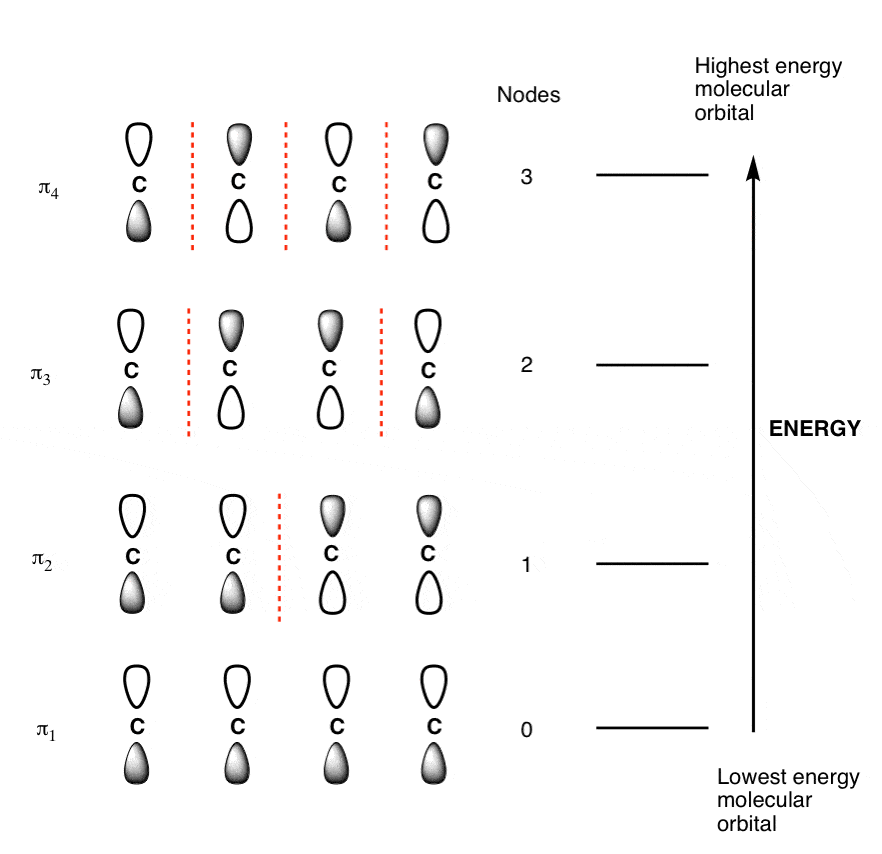
The Butadiene Pi System has zero nodes at its lowest energy molecular orbital.
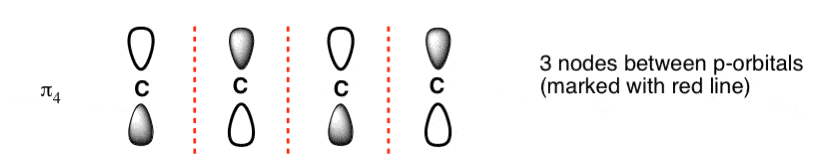
The Butadiene Pi System has three nodes at its lowest energy molecular orbital. The drawn n-p orbital are at alternate phase to each other. This create the a pi system with three nodes.
The Butadiene second lowest energy molecular orbital has one node. 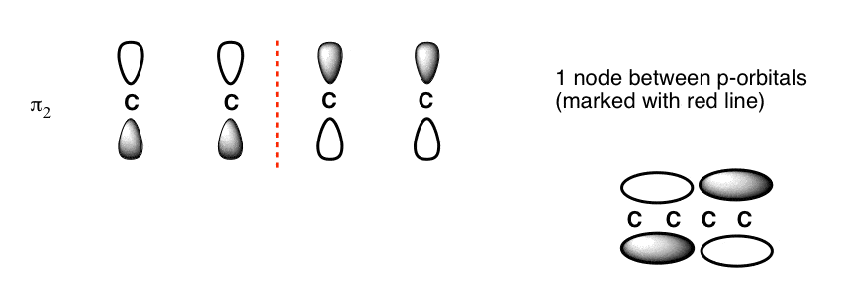
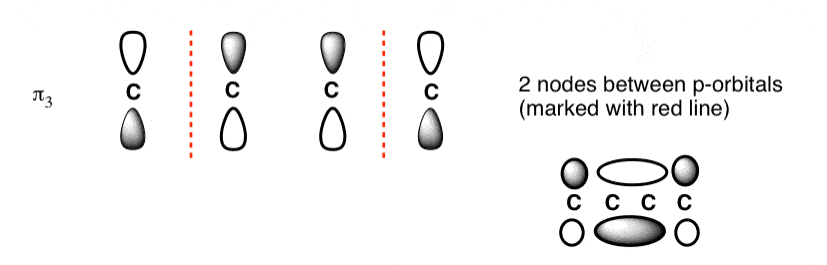
The third lowest energy molecular orbital has two nodes.
The Full Molecular Orbital Diagram For The Butadienyl System.
 Populating The Molecular Orbitals Of Butadiene With Electrons
Populating The Molecular Orbitals Of Butadiene With Electrons
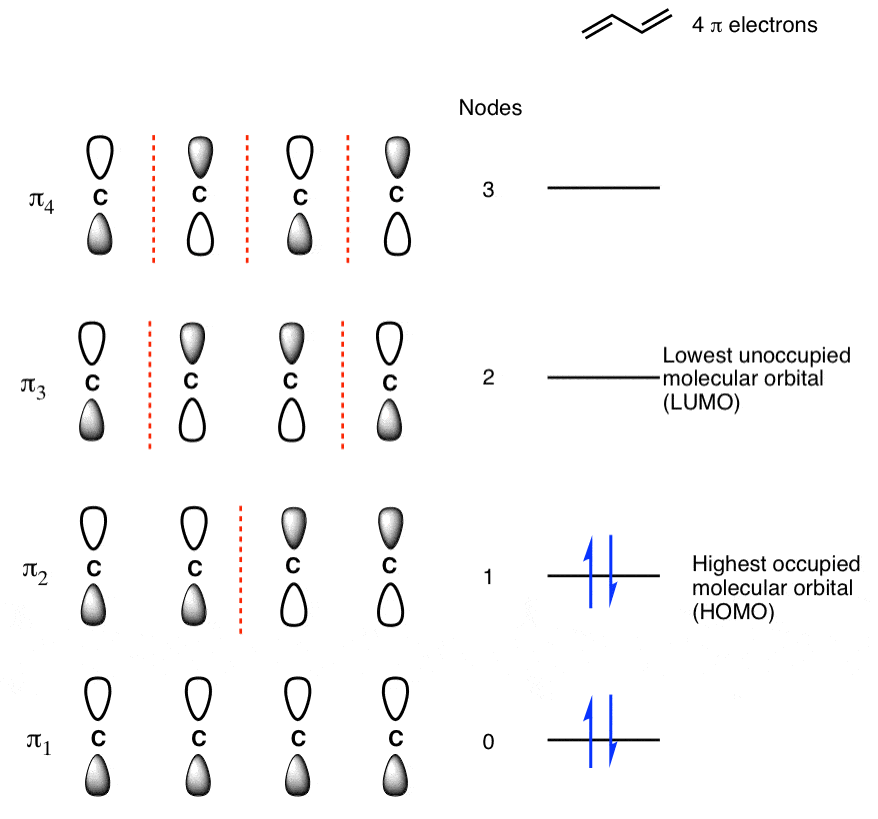
Q-5 Explain the Pi molecular orbital of benzene.
Answer: π molecular orbital of benzene:
The Pi molecular orbital diagram for Benzene
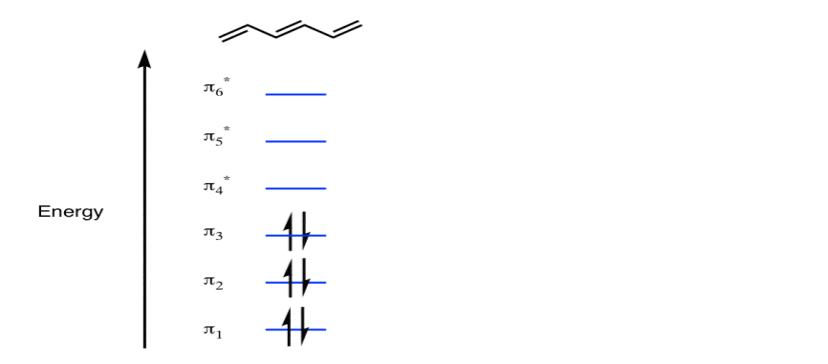
In the above increasing energy level the bottom three orbital are all bonding orbital while the top 3 orbital are anti bonding orbital.
The Benzene System has zero nodes at its lowest energy molecular orbital.
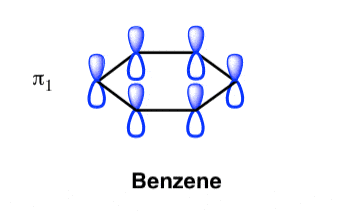
Benzene Has Nodal Planes. The Maximum Energy Level Has 3 Nodal Planes
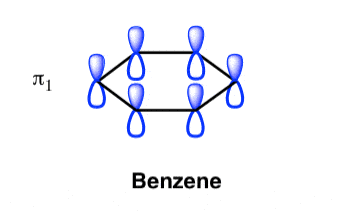
This orbital has zero overlap between adjacent p orbitals and therefore electrons in this orbital have the minimum possible delocalization. They are therefore the highest energy.
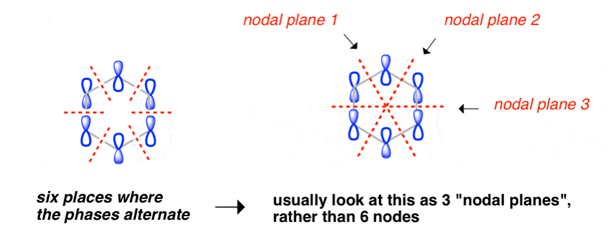
The pi molecular orbital of benzene
Q-6 Why does splitting affected? Mention in points.
Answer: The splitting can be affected by following factors:-
• Metal ion nature
• Metals oxidation state.
• Ligand arrangement around the metal ion
• Metal coordination number
Q-7 Explain spectrochemical series.
Answer: Spectrochemical Series: The ability of ligands to cause a large splitting of the energy between the orbital is essentially independent of the metal ion and the spectrochemical series is a list of ligands ranked in order of their ability to cause large orbital separations.
I- < Br- < SCN- ~Cl- < F- < OH- ~ ONO- < C2O42- < H2O< NCS- < EDTA4-<NH3 ~ pyr ~ en < bipy < phen < CN- ~ CO
Q-8 Hoe does N-type material effect the doping.
Answer: The effect of doping on an N-type material is as follows −
Q-9 How does P-type material effect doping?
Answer: The effect of doping on a P-type material is as follows −