(i) Ion-dipole interaction: The force that exerts between the cation or anion with presence of another polar molecule. E.g. It can be seen in HCl. The Hydrogen and Cl of same molecule are bonded with ionic bond while it is further attached to other component of same element with Vander Waal Forces.
(ii) Dipole-Dipole Interaction: This is the interaction between 2 different polar molecules. This is one of the second strongest Vander Waal Forces.
(iii) Ion-Induced Dipole Interaction: In the type of Vander Waal interaction one molecule is ionic while other one is non-polar molecule. The non-polar molecule induced by the effect of first element.
(iv) Dipole-Induced Dipole Interaction: In this type of Vander Waal interaction there are one polar and other one non-polar molecule. So the polar molecule makes the non-polar molecule to be polarized.
(v) Instantaneous Dipole-Induced Dipole Interaction: In this the both molecule are non-polar. The first molecule is become polarized instantly on the condition when the electron of a molecule revolving around the nucleus and at the instant time at which all the electron are oriented at a single place and hence that tend to result in the formation of another non-polar molecule to be polarized. This is also called as the London Forces or Dispersion Forces.
Q6) Enlist the characteristics of Vander Waal Forces.A6) The main characteristics of van der Waals forces are:
|
|
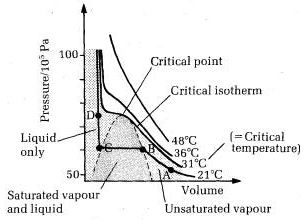
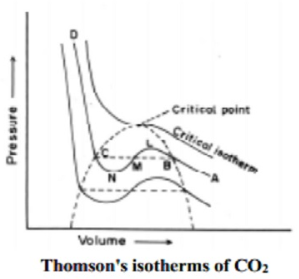
A8) For a real gas, using Vander Waals equation, the volume of a real gas is given as (Vm – b), where b is the volume occupied by per mole. Therefore, ideal gas law when substituted with V = Vm – b is given as: P(Vm−b)=nRT Because of intermolecular attraction P was modified as below (P+aV2m)(Vm−b)=RT (P+an2V2)(V−nb)=nRT Where, Vm: molar volume of the gas R: universal gas constant T: temperature P: pressure V: volume Thus, Vander Waals equation can be reduced to ideal gas law as PVm = RT. Vander Waals Equation Derivation for one mole of gas: Following is the derivation of Vander Waals equation for one mole of gas that is composed of non-interacting point particles which satisfies ideal gas law: p=RTVm =RTv p =RTVm−b C =NaVm (proportionality between particle surface and number density) a′C2=a′(NA/VM)2 =a/V2m p =RT/(Vm−b)−(a/V2m) ⇒(p+aV2m)(Vm−b) =RT (p+n2a/V2)(V−nb) =nRT (substituting nVm = V) Van der Waals equation applied to compressible fluids: Compressible fluids like polymers have varying specific volume which can be written as follows: (p+A)(V−B)=CT Where, p- pressure V- specific volume T- temperature |
A10) Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system. G=H-TS Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.
While at constant temperature this reaction transform into:
The Nernst Equation is derived from the Gibbs free energy under standard conditions. E*=E*reduction-E*oxidation ………..(i)
Where, n=no. of transferred electrons in the reaction F= Faraday constant E=Potential Difference. While when we see in the standard condition then, equation (ii) becomes
Hence, Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.
−nFE=−nFEo+RTlnQ …………….(v) On Dividing both sides of the Equation above by −nF, E=E*−RTnFlnQ(6) ……….(vi) Equation (vi) in the form of log10: E=E*−2.303RT/nF log10Q …….(vii) At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation (vii) can be rewritten: E=E*−0.0592V/n log10Q ……..(viii)
Then on substituting the these values to Nernst Equation we get, 0=E*-RT/nF In K …….(ix) At room temperature it becomes; 0=E*-0.0592V/n Log10K LogK=nE*/0.0592V The above equation clearly indicates the equilibrium constant K is proportional to the standard potential. |