Unit IV
Thermodynamics
Question Bank
Question 1) Explain Preventive Maintenance of Boilers?
Answer 1)
Daily check list
Blow down and test low water cut off
Blow down gauge glasses
Blow down boiler
Check boiler and system for leaks
Check burner flame
Weekly checklist
Check flame signal strength for both pilot and main
Check pilot and main fuel valve shutoff valves closing
Check ignite and burner operation
Check level in chemical treatment tank
Monthly checklist
� Check boiler water treatment test results and adjust if necessary
� Lubricate motors and equipment bearings
� Check main fuel safety shut off valve for leakages
� Check low fire start interlock
� Check high pressure/temperature interlocks
� Check high and low pressure interlocks on gas train
� Manually lift safety valve by hand
Six-monthly checklist
� Inspect burner components
� Check flame failure system components
� Check piping and wiring of all interlocks and shut off valves
� Recalibrate all instruments, indicating and recording gauges
� Perform a slow drain test for low water cut off
� Check combustion control system
� Check oil atomizer nozzles and strainers
� Test boiler safety valve as per ASME standards
Annual checklist
� Perform the six monthly checks
� Check all equipment coils and diaphragm
� Perform a pilot turn down test
� Recondition or replace low water cut off assembly
� Check gas drip leg and gas strainer
� Clean boiler fireside
� Drain boiler open hand holes and manholes and clean water side
� Have boiler inspected by a competent & authorized boiler inspector
� Clean burner blower primary and secondary fans
� Replace all gaskets
� Leak test all fuel valves
� Test operation of all controls and safety devices
� Adjust combustion parameters for low and high fire conditions
� Retest and re certify boiler monitoring system
Question 2) What is combustion efficiency in steam boiler?
Answer 2)
Managing your boiler with the best amount of excess air will reduce heat loss up to the mass and enhance combustion efficiency. Combustion efficiency is a measure of how efficiently the heat content of a fuel is transported into valuable heat. The stack temperature and flue gas oxygen combinations are primary symbols of combustion efficiency.
The combustion efficiency of a boiler signifies the burner’s capacity to burn fuel. It has two parameters that determine the burner efficiency: unburnt fuel supplies in the exhaust and excess oxygen levels in the exhaust.
As the value of excess air is increased, the amount of unburnt fuel in the exhaust decreases. This, in the end, lowers the unburnt fuel failures but raise the enthalpy losses. This is why it is very important to keep a balance between enthalpy losses and unburnt losses. Combustion efficiency also alters with the fuel being consumed. The combustion efficiency is higher in case of for liquid and gas fuels and is lower for solid fuels.
Question 3) Explain Heat Losses in Boiler?
Answer 3)
There are various losses after combustion of fuel in the boiler.
(1) Heat Loss Due To Dry Flue Gas:
This is the highest loss in boiler.
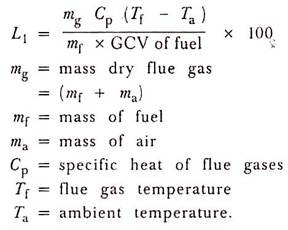
(2) Heat Loss Due to Evaporation of Water Formed Due to H2 in fuel:
The combustion of hydrogen causes heat loss because of the product of combustion is water.
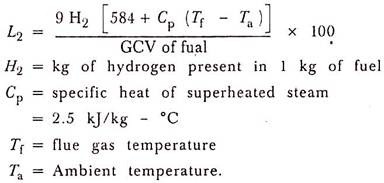
(3) Heat Loss Due to Moisture Present in Fuel:
The moisture entering the boiler with the fuel leaves as a superheated vapour.
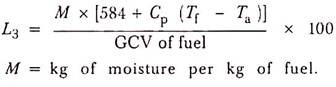
(4) Heat Loss Due to Moisture Present in Air:
Vapour is present in from of humidity in the incoming air. The mass of vapour which air contains can be obtained from psychrometries charts.
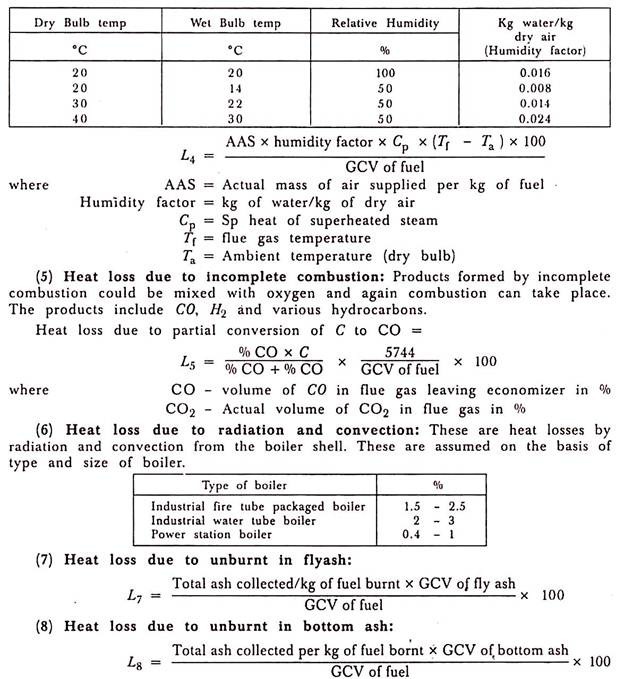
Question 4)Difference Between Fire Tube Boiler and Water Tube Boiler:
Answer 4) Here are the 15 points on differences between Fire and Water-tube boiler:
FIRE TUBE BOILER | WATER TUBE BOILER |
Hot flue gases flow inside the tube and the water outsides the tubes. | Water flows inside the turbine and hot flue gases outside the tube. |
These boilers are generally internally fired. | These boilers are generally extra really fired. |
The boiler pressure limited to 20 bar. | The boiler pressure is limited to up to 100 bar. |
The fire-tube boiler has a lower rate of steam production. | A higher rate of steam production. |
Not suitable for larger power plants. | Suitable for larger power plants. |
Involves lesser risk of explosion due to low pressure. | The risk of the explosion is higher due to high boiler pressure. |
For a given power, it occupies large floor space. | For a given power, it occupies small floor space. |
This boiler is difficult to construct. | Simple in construction. |
9. | Difficult in transportation. | Simple in transportation. |
10. | They require less skill to operate, as compare to the water tube boiler. | They required a skilled operator. |
11. | They are difficult to repair and cleaning as they are internally fired. | They are easy to repair and clean as they are externally fired. |
12. | They required a large shell diameter. Because the firetube situated inside the shell. | They required a small shell diameter. |
13. | The efficiency of the fire tube boiler is less as compared to the water tube boiler. | The efficiency of the water tube boiler is more. |
14. | The maintenance of this boiler is costly. It requires regular inspection. | They are easy to maintain as they are externally fired. |
15. | Ex: Cornish Boiler, Lancashire Boiler. | Ex: Babcock and Wilcox Boiler. |
Question 5) Explain the Necessity of Boiler?
Answer 5)
The most common function for any boiler, whether it is an industrial or residential boiler, is to serve as the central heating mechanism for a home, business facility, hospital, commercial complex, etc.
No matter what setting they are used in, boilers operate with the same basic functions and mechanisms that work together to create a contained, heat-generating combustion process.
Boilers draw natural gas from gas lines running through our streets and use this gas to fuel the combustion process for heat creation and distribution throughout a building.
The boiler system relies on a burner to initiate the combustion process, and then heat in the form of steam or hot water moves through the system using pumps, radiators, and heat exchangers.
Boiler manufacturers are making use of rapidly improving technology to build equipment that is cost-efficient, environmentally friendly, and powerful.
Question 6)Explain Draught Losses?
Answer 6)
When the ash pit door is closed and there is no flue gas flow. Under this condition a draught gauge located at the grate level at the base of the chimney will read the total static’ draught which the chimney develops. When flue gas is flowing the grate level draught gauge reading will be less than the total static draught and the difference in two readings gives the drop in draught.
The drop in draught is caused by:
(i) Frictional resistance offered by the flues and gas passages to the flow of flue gases
(ii) Flow of flue gases at various bends in the gas circuit, and
(iii) Imparting of velocity to the flue gases.
Due to the drop or loss in draught we get another term known as available draught. The available draught at any point along the flue gas circuit is the draught which is read by means of a draught gauge located at the point under consideration. Thus, we get the relation available draught = total static draught – total draught drop.
The draught necessary for a given boiler plant depends on:
(i) To overcome friction in flue,
(ii) To overcome the resistance offered to the flow of gases by the baffle walls and boiler tubes,
(iii) To force the flue gas through economizer, air pre-heater, etc.,
(iv) To force the air through the fuel bed on the grate,
(v) To overcome the friction loss in the chimney, and
(vi) To impart velocity to gas in the chimney.
The draught required to overcome friction in flue is 10 mm of water column for 100 metre length and the drop in draught at each right-angle bend is 2 mm of water column. The draught required to overcome the resistance offered to flow of gases in the boiler tubes and baffles depends on the kind of boiler used and type of baffles, and on mass flow. It varies from 5 to 40 mm of water column.
Question 7)Explain the Definition of Steam Terms?
Answer 7)
Heat – A form of energy
Temperature – The degree of hotness with no implication of amount of heat energy. Expressed in terms of degrees Fahrenheit: 32° is freezing, 212° is the boiling point at atmospheric pressure.
BTU - (British thermal unit) The amount of heat energy required to raise the temperature of one pound of water 1°. More correctly, 1/180 of the amount of heat required to raise 1 pound of water from 32° to 212° at atmospheric pressure.
Pressure - The collision of molecules of a gas with the walls of a container, represented in PSI, PSIA, or PSIG.
PSI: Pounds per square inch
PSIA: Pounds per square inch absolute - includes atmospheric pressure (0 PSIG=14.7 PSIA)
PSIG: Pounds per square inch gauge – measures pressure above atmospheric
Sensible Heat – Expressed in BTU/lb. The heat required to bring one lb. Of water from the freezing point to boiling point corresponding to any pressure. Higher pressures mean higher boiling points.
Latent Heat of Evaporation – Expressed in BTU/lb. The amount of heat required to convert one pound of water to steam. Latent heat is the potential energy of steam or the useable part of steam. When these BTUs are released or given up, condensation takes place and a pound of condensate results. Note that as pressure increases, the BTU requirement to change one pound of water to steam decreases.
Specific Volume – Expressed in ft3/lb. The space occupied by one pound of steam at a particular pressure. As pressure increases, specific volume decreases
Question 8) Explain the Types of Steam?
Answer 8)
Steam is an invisible gas created by adding heat energy to water. It is liquid water changed to its gaseous state.
Saturated steam - steam in immediate contact with the water from which it is being generated. If the pressure remains constant, any loss of heat or BTUs will result in condensation.
Superheated steam – If more heat is added to dry saturated steam at a constant pressure, increasing its temperature and specific volume, super-heated steam is produced. Heat must be lost, and temperature reduced before condensation occurs.
Flash steam - when condensate, at saturation temperature and pressure, is discharged into a region of lower pressure, it automatically adjusts to the saturated conditions at the lower pressure. In effect, some of the condensate is “re-evaporated” into steam.
Question 9) What do you mean by the term state in thermodynamics?
Answer 9) State:
The application of thermodynamic principles begins by defining a system that is in some sense distinct from its surroundings. For example, the system could be a sample of gas inside a cylinder with a movable piston, an entire steam engine, a marathon runner, the planet Earth, a neutron star, a black hole, or even the entire universe. In general, systems are free to exchange heat, work, and other forms of energy with their surroundings.
A system’s condition at any given time is called its thermodynamic state. For a gas in a cylinder with a movable piston, the state of the system is identified by the temperature, pressure, and volume of the gas. These properties are characteristic parameters that have definite values at each state and are independent of the way in which the system arrived at that state. In other words, any change in value of a property depends only on the initial and final states of the system, not on the path followed by the system from one state to another. Such properties are called state functions. In contrast, the work done as the piston moves and the gas expands and the heat the gas absorbs from its surroundings depend on the detailed way in which the expansion occurs.
The behaviour of a complex thermodynamic system, such as Earth’s atmosphere, can be understood by first applying the principles of states and properties to its component part in this case, water, water vapour, and the various gases making up the atmosphere. By isolating samples of material whose states and properties can be controlled and manipulated, properties and their interrelations can be studied as the system changes from state to state.
Question 10)Explain the difference between heat and work?
Answer 10)
WORKHEAT
Interaction | Mechanical | Thermal |
Requires | Force and Displacement | Temperature difference |
Process | Macroscopic pushes and pulls | Microscopic collisions |
Positive value | W > 0 when a gas is compressed. Energy is transferred into system. | Q > 0 when the environment is at a higher temperature than the system. Energy is transferred into system. |
Negative value | W < 0 when a gas expands. Energy is transferred out of system. | Q < 0 when the system is at a higher temperature than the environment. Energy is transferred out of system. |
Equilibrium | A system is in mechanical equilibrium when there is no net force or torque on it. | A system is in thermal equilibrium when it is at the same temperature as the environment. |