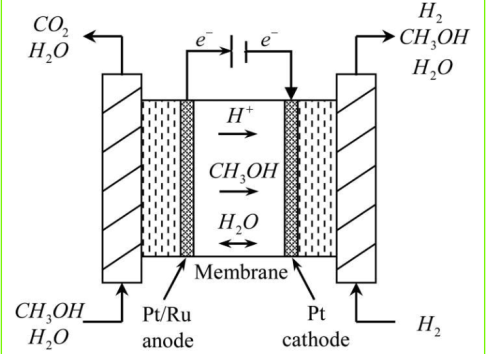
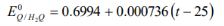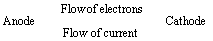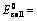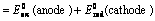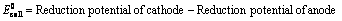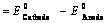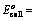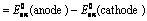|
The Cu2+/Cu redox couple (corresponding to the reaction Cu2+(aq) + 2e– → Cu (s) ) is represented by Cu (s) | Cu2+(aq) The Fe3+/Fe2+ redox couple (corresponding to the reaction Fe3+(aq) + e– → Fe2+(aq) ) is represented: Pt | Fe2+(aq) , Fe3+(aq)
|
Cu (s) | Cu2+(aq) || Fe3+(aq) , Fe2+(aq) | Pt
|
2Fe3+(aq) + Cu (s) → 2Fe2+(aq) + Cu2+(aq) |
EoCell=EoRed,Cathode−EoRed,Anode |
Q3)Explain Calomel Quinhydrone Electrode? A3) Calomel electrodes is used in voltmeter and also pH meters, it’s a kind of reference electrode, and is based on reactions between mercury(I) chloride (calomel) and elemental mercury.A calomel electrolyte should be a non-polarizable one and robust, they are commonly used two-electrode systems, where the supporting electrolyte is a non-reactive chloride salt. The structure of the electrode includes of an outer glass with a frit at the bottom, which allows electric contact with a solution outside the electrode. An inner tube is present inside an outer tube. The bottom of the inner tube has glass wool to enable electric contact between the contents of both tubes.On the innermost tube, is the Mercury paste, with mercurous chloride being dispersed in a saturated potassium chloride solution.
|
ΔG =-nFE Where, n = number of electrons transferred in the reaction, ΔG= Gibbs free energy, E= cell potential F = Faradays constant (96,500 C/mol) and. Under standard conditions, the above equation can be written as, ΔGo =-nFEo According to the theory of thermodynamics, Gibbs free energy under general conditions can be related to Gibbs free energy under standard condition and the reaction quotient as: ΔG=ΔGo + RT lnQ Where, Q= reaction quotient, R= universal gas constant and T= temperature in Kelvin. Incorporating the value of ΔGo and ΔG, from the first two equations, we get the equation: -nFE = -nFE0 + RT lnQ E = E0 – (RT/nF) lnQ By conversion of Natural log to log10, the above equation is called as the Nernst equation. Here, it shows the relation of the reaction quotient and the cell potential. Special cases of Nernst equation: E = Eo − (2.303RT/nF) log10Q At standard temperature, T= 298K: E = Eo − (0.0592V/n) log10Q At standard temperature T = 298 K, the 2.303RTF, term equals 0.0592 V. |
E0 – (2.303RT/nF) log10Keq = 0 E0 = (2.303RT/nF) log10Keq |
|
(ii) When reduction potentials of both electrodes are taken into consideration
|
|
|
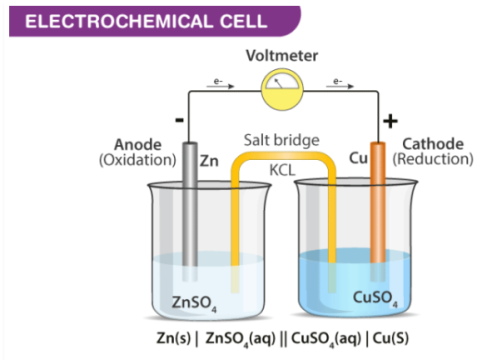
Cathode | Anode |
Cathode has a positive sign, as electrons are consumed | Anode have a negative sign as electrons are liberated here |
In an electrochemical cell, at the cathode a reduction reaction occurs. | At the Anode an oxidation reaction occurs |
Electrons move into the cathode | Electrons move out of the anode |