Unit - 15
Repairs & Rehabilitation of Structures
Q1) Define physical inspection of damaged structure?
A1) Some of the use full information may be obtained from the physical inspection of damaged structure, like nature of distress, type of distress, extent damage and its classification etc, their causes preparing and documenting the damages, collecting the samples for laboratory testing and analysis, planning for in situ testing, special environmental effects which have not been considered at the design stage and information on the loads acting on the existing structure at the time of damage may be, obtained. To stop further damages, preventive measure necessary may be planned which may warrant urgent execution.
Q2) How deterioration occurs due to corrosion?
A2)
Spelling of concrete cover
Cracks parallel to the reinforcement
Spelling at edges
Swelling of concrete
Dislocation
Internal cracking and reduction in area of steel reinforcement
Q3) What are the steps in selecting a repair procedure?
A3)
Consider total cost
Do repair job in time
If defects are few & isolated repair on an individual basis Otherwise do in generalized manner.
Ensure the repair prevents further development of defects
In case of lost strength, repairs should restore the strength
If appearance is a problem, the number of applicable types of repairs become limited the repairs must be covered
Repair works should not interface with facilities of the structure
Take care in addition of section to a member and in reattributing live loads and other live load moments. After selecting a suitable method of repairs, and after considering all the ramifications of its application, the last step is to prepare plans & specification and proceed with the work.
Q4) Discuss about the design and construction errors leading to deterioration of a structure?
A4) Design of concrete structures governs the performance of concrete structures. Well designed and detailed concrete structure will show less deterioration in comparison with poorly designed and detailed concrete, in the similar condition. The beam-column joints are particularly prone to defective concrete, if detailing and placing of reinforcement is not done properly. Inadequate concrete cover may lead to carbonation depth reaching up to the reinforcement, thus, increasing the risk of corrosion of the reinforcement.
Q5) What are the techniques required for repairing cracks?
A5)
Bonding with epoxies
Routing and sealing
Stitching
Blanketing
External stressing
Grouting
Autogenous healing
Q6) Difference between defects, distress and deterioration Defects?
A6) The defects are the flaws those creeps into structure because of design mistakes or poor workmanship during manufacturing, fabrication and construction, before it begins its service life, or by inappropriate operation and maintenance during its service life. The flaw that has a potential to lead to a failure, becomes a defect.
Distress: It is a collective term for the physical manifestation of problems such as cracks, spalls, pop-outs, staining, decay or corrosion. Distress can be thought of as the symptoms indicating that the defects are present.
Deterioration: It is the gradual loss of the desired material properties due to different degradation factors. Deterioration unlike defects, may not surface at the beginning of the service life of a structure, but is rather time-dependent. However, some forms of deterioration may develop early in the service life of structure and others manifest later.
Q7) Explain ASM classification of corrosion type?
A7) 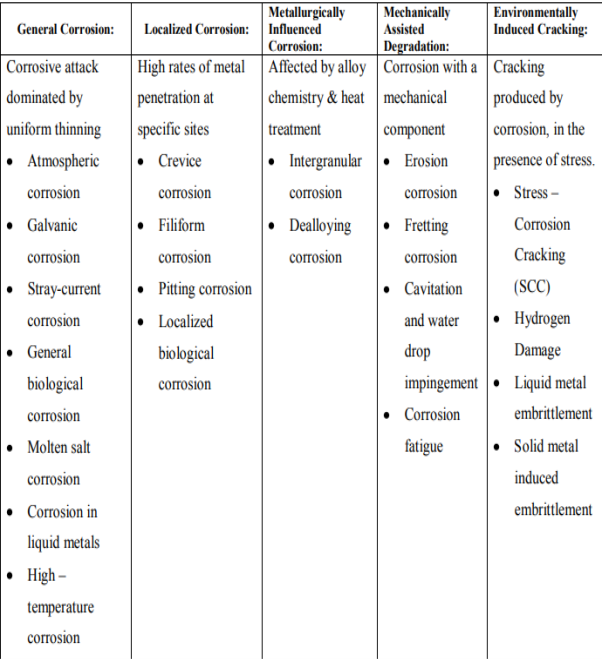
Q8) Explain Mechanism of Corrosion?
A8) The corrosion process that takes place in concrete is electrochemical in nature very similar to a battery. The mechanism of corrosion involves four basic elements
Anode: Site where metal atoms lose electrons i.e., where corrosion is initiated.
Cathode: Site where electrons flow to and combine with other metallic and non-metallic ion.
Electrolyte: A medium capable of conducting electric current by ionic current flow.
Metallic path: Connection between the anode and cathode that completes the circuit.
Corrosion is an irreversible interfacial reaction of a material (metal, ceramic, polymer) with its environment which results in its consumption or dissolution into the material of a component of the environment. Often, but not necessarily, corrosion results in effects detrimental to the usage of the material considered. Exclusively physical or mechanical processes such as melting and evaporation, abrasion or mechanical fracture are not included in the term corrosion” With the knowledge of the role of various microorganisms present in soil and water bodies, the definition for corrosion need be further widened to include microbially-influenced factors.
Corrosion can be classified in different ways, such as
Dry corrosion occurs in the absence of aqueous environment, usually in the presence of gases and vapours, mainly at high temperatures. Electrochemical nature of corrosion can be understood by examining zinc dissolution in dilute hydrochloric acid.
Zn + 2HCl = ZnCl2 + H2
Anodic reaction is Zn = Zn++ + 2e with the reduction of 2H+ + 2e = H2 at cathodic areas on the surface of zinc metal.
Q9) Explain GALVANIC (BIMETALLIC) Corrosion?
A9) When two dissimilar metals are immersed in a conducting solution, they usually develop different corrosion potentials. If the metals are in contact this potential difference provides the driving force for increased corrosion, the less noble of the two metals corroding more rapidly, while the more noble corrodes less.
An electrochemical series based on the standard thermodynamic data for the metals is frequently used as a basis for ranking metals under these conditions, but a more practical means of ranking is a galvanic series determined experimentally for particular corrosion elements.
Dissimilar metal contacts provide the first example of situations in which the anodic and cathodic areas of a corrosion couple can be separated and therefore the rates of the two reactions may be substantially different, while electro
Neutrality is still maintained. Although tables of galvanic behaviour will show which alloy of a galvanic couple will become anodic and which cathodic, they give no indication of the rate of corrosion to be expected of the anodic material. For example, many active/passive materials which behave well in sea water and would be expected to be cathodic in many couples are in fact not very good cathodes and readily become polarised. Under these conditions’ acceleration of attack of the baser member of the couple is often less than might be expected. The prediction of rates is thus quite complex, and there is much qualitative tabular material available to provide assistance
Base Magnesium
Zinc
Aluminium (commercial)
Cadmium
Duralumin (Al with 4½% Cu)
Mild steel
Cast iron
Stainless steel (Type 430; 18% Cr) ACTIVE
Stainless steel (Type 304; 18% Cr 10% Ni) ACTIVE
Lead-tin solders
Lead
Tin
Nickel
Brasses
Copper
Bronze
Monel
Silver solders (70% Ag 30% Cu)
Nickel PASSIVE
Stainless steel (Type 430) PASSIVE
Stainless steel (Type 304) PASSIVE
Silver
Titanium
Graphite (Carbon) (non-metal)
Gold
Noble Platinum
Q10) What is Crevice Corrosion?
A10) It is quite possible for corrosion to become localised on a single metal if environmental conditions are able to develop non-uniformly over the surface. The existence of crevices, either as a result of design or by development of deposits etc, can lead to occluded regions in which oxygen, the usual cathodic reactant, cannot be replenished. Whereas both anodic and cathodic reactions once occurred uniformly over the surface, after a short period the only reaction remaining in the crevice may be the anodic reaction, balanced by cathodic action outside the crevice. The resulting crevice corrosion is most serious in active/passive materials where a sequence of events may follow leading to significant and irreversible changes in the environment within the crevice.