Unit - 4
Biomolecules
Q1) Define Biomolecules?
A1) The basic unit of life is the cell. All organisms consist of one or more cells. In humans many millions of cells are present.
The cells of all organisms consist of indivisible units of life they have four fundamental macromolecular components namely: nucleic acids (including DNA and RNA), proteins, lipids and glycans. The modification and interaction these of components results in the cell development and functions.
Each cell in the human body is minute and simple form of life. Each type of cell is programmed to do different things, such as carry oxygen, or defend the human body against viruses and bacterial infections and transmit signals to the brain. The origin of cells is linked directly to the origin of life
Since they are present in living things these building blocks are called biomolecules.
Q2) Differentiate a monomer and a polymer?
A2) A monomer is the basic unit that chemically binds to other molecules to form a polymer. In case of proteins, the monomers are amino acids. For lipids, the monomers are glycerol and fatty acids for nucleic acids, the monomers are nucleotides which is made of a nitrogenous base, a pentose sugar and a phosphate group. The function of a monomeric structural unit is defined as the number of covalent bonds that are formed with other reactants. A structural unit in a linear polymer chain segment forms two bonds and is therefore bifunctional. A monomer is a type of molecule that has the ability to chemically bond with other molecules in a long chain; a polymer is a long chain of an of consisting unspecified number of monomers. Essentially, in polymers, monomers are the building blocks, and polymers are more complex type of molecules. Monomers—repeating molecular units—are connected into polymers by bonds that are called as covalent bonds
The word monomer is derived from mono- (one) and -mer (part). Monomers are small molecules which are bound closely together in a repeating manner to form more complex molecules called polymers. Monomers form polymers by forming chemical bonds or binding molecules through a process called polymerization. One of the most abundant natural polymers is Glucose, which polymerizes by forming glycosidic bonds.
A polymer is derived from (Greek poly "many" + mer, "part") is a large molecule, or macromolecule, composed of several repeated subunits. Due to their broad range of properties, both synthetic and natural polymers play essential and specific roles in daily life. Polymers range from natural biopolymers such as DNA and proteins that are fundamental to biological structure and function to synthetic plastics such as polystyrene. Polymers, both synthetic and natural, are formed through polymerization of many smaller molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, viscoelasticity, and a tendency to form glasses and semi crystalline structures rather than crystals. Polymers are studied widely in the fields of macromolecular science and biophysics, and polymer science. Historically, by observation the products arising from the linkage of repeating units by covalent chemical bonds have been the primary focus of polymer science; emerging important areas of the science now focus on non-covalent links. Polyisoprene of latex rubber is an example of a biological polymer, and the polystyrene of Styrofoam is an example of a synthetic polymer. In biological contexts, essentially all biological macromolecules—i.e., proteins (polyamides), nucleic acids (polynucleotides), and polysaccharides—are purely polymeric in nature.
Q3) Write a short note on Sugar?
A3) In chemical terms, “sugar” usually refers to all carbohydrates with the general formula Cn (H2O) n. Sucrose is a disaccharide molecule, or double sugar, that is composed of one molecule of glucose linked to one molecule of fructose. As one molecule of water (H2O) is lost in the condensation reaction linking glucose to fructose, sucrose is represented by the formula C12H22O11 (following the general formula Cn [H2O] n - 1).
Sucrose is predominantly present in all plants, but is known to occur at high concentrations that is sufficient for economic recovery only in sugarcane , it is a sweet crystallizable material that consists essentially or wholly of sucrose, is colourless or white when it is in pure and tends to be brown when less refined, is obtained commercially from sugarcane or sugar beet and less extensively from palms, maples and sorghum and is important as a source of dietary carbohydrate and is also used as a sweetener and preservative for a variety of foods and of various water-soluble compounds that differ widely in sweetness, including the monosaccharides and oligosaccharides, and typically are optically active.
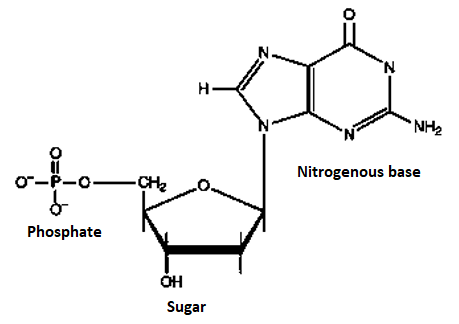
Q4) Define Nucleotides?
A4) Nucleotide, any member or group of a class of organic compounds, that have a molecular structure that comprises a nitrogen-containing unit (base) linked to a sugar and a phosphate group. The nucleotides are of great importance to living organisms, as they form the building blocks of nucleic acids, nucleic acids are the substances that control all hereditary characteristics
In the two families of nucleic acids, Ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), the sequence of nucleotides in the RNA or DNA codes for the structure of proteins synthesized in the cell. The driving force for many metabolic processes is supplied by the nucleotide adenosine triphosphate (ATP). Several nucleotides also act coenzymes; they combine with enzymes to speed up (catalyse) biochemical reactions. Nucleotide structure is simple, but the structure they can form together with other compounds is complex. Each nucleotide within has a specific structure which is shown below.
Q5) Explain the differences between DNA and RNA?
A5)
Comparison | DNA | RNA |
Full Name | Deoxyribonucleic Acid | Ribonucleic Acid |
Function | DNA replicates and stores genetic information. The special feature is it is the blueprint for all genetic information contained within an organism | RNA converts the genetic information contained within DNA to a format used to build proteins, and then moves it to ribosomal protein factories. |
Structure | DNA consists is double stranded, arranged in a double helix. These strands are made up of subunits called nucleotides. Each nucleotide contains a phosphate, a nitrogenous base and a 5-carbon sugar molecule. | RNA only is single stranded, but like DNA, strand is made up of nucleotides. RNA strands are shorter than DNA strands. RNA sometimes forms a secondary double helix structure, but only occasionally. |
Length | DNA is a much longer polymer than RNA. A chromosome, for example, is a single, long DNA molecule, when opened would be several centimetres in length. | RNA molecules vary in length, but are found to be much shorter than long DNA polymers. A large RNA molecule may be a thousand base pairs in length. |
Sugar | The sugar in DNA is deoxyribose, which contains one less hydroxyl group than RNA’s ribose. | RNA contains ribose sugar molecules, without the hydroxyl modifications of deoxyribose. |
Bases | The bases in DNA are Adenine (‘A’), Thymine (‘T’), Guanine (‘G’) and Cytosine (‘C’). | RNA shares Adenine (‘A’), Guanine (‘G’) and Cytosine (‘C’) with DNA, but contains Uracil (‘U’) rather than Thymine. |
Base Pairs | Adenine and Thymine pair (A-T) Cytosine and Guanine pair (C-G) | Adenine and Uracil pair (A-U) Cytosine and Guanine pair (C-G) |
Location | DNA is found in the nucleus, with a small amount of DNA also present in mitochondria. | RNA forms in the nucleolus, and then moves to specialised regions of the cytoplasm depending on the type of RNA formed. |
Reactivity | Due to its deoxyribose sugar, which contains one less oxygen-containing hydroxyl group, DNA is a more stable molecule than RNA, which is useful for a molecule which has the task of keeping genetic information safe. | RNA, containing a ribose sugar, is more reactive than DNA and performs enormous tasks but is not stable in alkaline conditions. RNA’s larger helical grooves mean it is more easily affected by the attack of enzymes. |
Ultraviolet (UV) Sensitivity | DNA is vulnerable to damage by ultraviolet light. | RNA is more resistant to damage from UV light than DNA. |
Q6) Explain lipids in detail?
A6) Lipid, are a diverse organic group of compounds including fats, oils, hormones, and certain components of membranes that are grouped together because they do not interact well with water. The triglycerides are one type of lipids, when sequenced to form fat in adipose cells, and also serve as the energy-storage for organisms and also provide thermal insulation. Some lipids like steroid hormones serve as chemical messengers between organs, tissues and cells and other lipids communicate signals between biochemical systems inside a single cell. The organelles (structures present within the cell) and membranes of cells are found to be microscopically thin structures formed from two layers of phospholipid molecules. Organelles and membranes of cells function to separate individual cells from their environments and to categorize the cell interior into structures that carry out special functions. So important is this compartmentalizing function that membranes, and the lipids that help in forming them, must have been essential to the origin of life itself.
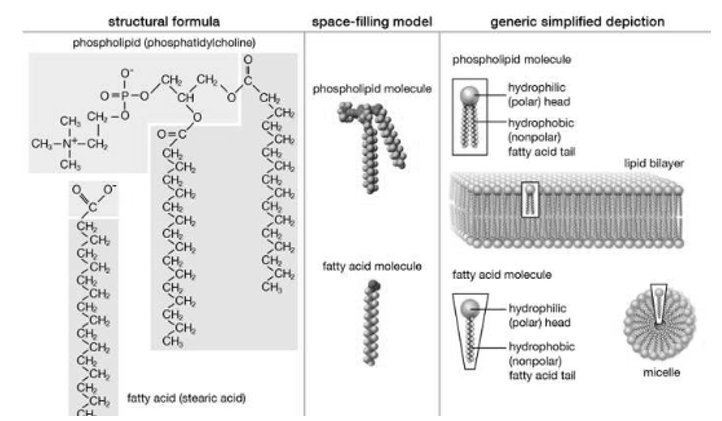
Structure of Lipid, Structure and properties of two representative lipids are shown in the picture. Both stearic acid which is a fatty acid and phosphatidylcholine which is a phospholipid, they form polar ‘heads’ and nonpolar ‘tails’ as they are composed of chemical groups. The polar heads are found to be hydrophilic, they are soluble in water, whereas the nonpolar tails are hydrophobic, they are insoluble in water. With this kind of composition in Lipid molecules they spontaneously form aggregate structures called micelles and lipid bilayers, the hydrophobic ends are however covered away from the water and the hydrophilic ends oriented toward the water.
Although biological lipids are not as large as macromolecular polymers (e.g., proteins, nucleic acids, and polysaccharides), many lipids are formed by the chemical linking of several small constituent of molecules. Many of these molecular building blocks are homologous, in structure. The homologies allow lipids to be classified into a few major groups: cholesterol and its derivatives, fatty acids, fatty acid derivatives and lipoproteins.
Q7) How are amino acids different from proteins?
A7) Amino acids are organic compounds in nature and combine to form proteins molecules. Amino acids and proteins are the building blocks of all lifeforms. When proteins are broken down or digested, amino acids are left. Compared to any other class of macromolecules, Proteins are among the most abundant organic molecules in living systems and are way more diverse in structure and function. A single cell can contain thousands of proteins, each having a unique function. All proteins are made up of one or more chains of Amino acids although their structures, like their functions. Proteins can play a wide array of roles in a cell or organism. The common protein is important in the biology of many organisms (including humans). Proteins come in many different shapes and sizes. Some are globular (roughly spherical) in shape, whereas others form long, thin fibers. For example, the haemoglobin protein that carries oxygen in the blood is a globular protein, while collagen, found in the skin, is a fibrous protein.
Amino acids are the monomers that make up proteins. Specifically, a protein is made up of one or more linear chains of amino acids, each of which is called a polypeptide. There are 20 different types of amino acids present in proteins
Amino acids share a basic structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (NH2) a carboxyl group {COOH}and a hydrogen atom.
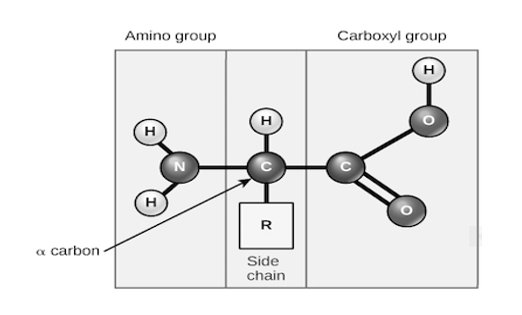
Basic structure of an amino acid, every amino acid also has another atom or group of atoms bonded to the central atom, known as the R group, which determines the identity of the amino acid. For instance, if the R group is a hydrogen atom, then the amino acid is glycine etc.
Q8) Write a short note on Cellulose?
A8) Cellulose a complex carbohydrate, or polysaccharide, consisting of 3,000 or more glucose units. They form the basic structural component of plant cell walls; cellulose comprises about 33 percent of all vegetable matter and is the most abundant naturally occurring organic compounds. Cellulose cannot be digested by man, cellulose is a source of food for herbivorous animals (e.g., cows, horses) because they retain it long enough for digestion by microorganisms present in the alimentary tract; protozoans in the gut of insects such as termites also digest cellulose. It has great economic importance, cellulose is processed to produce papers and fibres and is chemically modified to yield substances used in the manufacture of such items as plastics, photographic films, and rayon. Other cellulose derivatives are used as thickening agents for foods adhesives, explosives, and in moisture-proof coatings.
Q9) Define Denaturation?
A9) A protein’s shape is critical to its function, and, many different types of chemical bonds may be important in maintaining this shape. Changes in temperature and pH, as well as the presence of certain chemicals, may disrupt a protein’s shape and cause it to lose functionality, a process known as denaturation.
Denaturation is a process in which proteins or nucleic acids lose the quaternary structure, tertiary structure, and secondary structure which is present in their native state, by application of some external stress or compound such as a strong acid or base, a concentrated inorganic salt, an organic solvent (e.g., alcohol or chloroform), radiation or heat. Since denaturation reactions are not strong enough to break the peptide bonds, the primary structure (sequence of amino acids) remains the same after a denaturation process. Denaturation disrupts the normal alpha-helix and beta sheets in a protein and uncoils it into a random shape
Q10) Short notes on starch?
A10) Starch, is a granular, white, organic chemical that is produced by all green plants. Starch is a soft, white, tasteless powder that is insoluble in alcohol, cold water, or other solvents. The basic chemical formula of the starch molecule is (C6H10O5)n. Starch is a polysaccharide comprising of glucosemonomers that are joined in α 1,4 linkages. The simplest form of starch is the linear polymer amylose; amylopectin is the branched form.
Starch is manufactured in the green leaves of plants from the excess glucose produced during process of photosynthesis and serves as a reserve food supply for the plant. Starch is stored in chloroplasts of the cell in the form of granules and in others as storage organs in the roots of the cassava plant; the tuber of the potato; the seeds of corn, wheat, and rice and the stem pith of sago. According to the requirement, starch is broken down, in the presence of certain enzymes and water, into its constituent monomer glucose units, which diffuse from the cell to nourish the plant tissues. In humans and other animals, starch from plants is broken down into its constituent sugar molecules, which then supply energy to the tissues.