Unit - 7
Macromolecules
Q1) Define the Structure of Proteins?
A1) Proteins are very important molecules and building blocks that are essential for all living organisms. Proteins are the largest unit of cells when analyse by dry weight, each protein is eventually devoted to a specific function and Proteins are involved in virtually all cell functions, with tasks that range from locomotion and cell signalling and general cellular support. In total, there are seven types of proteins the structure of a protein may be fibrous or globular depending on its specific role (every protein is specialized). Globular proteins are generally soluble, compact, and spherical in shape. Fibrous proteins are typically elongated and insoluble. Globular and fibrous proteins may exhibit one or more types of protein structures.
There are four structural proteins: namely primary, secondary, tertiary, and quaternary. These levels aredetermined by the complexity that lies in the polypeptide chain this nature differentiates them from each other, the shape and function of a protein. The primary level is the most basic and rudimentary while the quaternary level demonstrates complex bonding.
The countless nature of a protein determines its specific structure and function. A single protein molecule may contain one or more of these protein structure level
Q2) Explain Proteins as Enzymes and Transporters?
A2) Enzymes are proteins that facilitate and speed up biochemical reactions, which is why they are often referred to as catalysts. Notable enzymes include lactase and pepsin, proteins that are familiar for their roles in digestive medical conditions and specialty diets. Lactose intolerance is caused by a lactase deficiency, an enzyme that breaks down the sugar lactose found in milk. Pepsin is a digestive enzyme that works in the stomach to break down proteins in food—a shortage of this enzyme leads to indigestion.
Other examples of digestive enzymes are those present in saliva: salivary amylase, salivary kallikrein, and lingual lipase all perform important biological functions. Salivary amylase is the primary enzyme found in saliva and it breaks down starch into sugar.
Enzymes regulate almost all the numerous and complex biochemical reactions that occurs in plants, animals, and microorganisms. These catalytic proteins are efficient and specific—which means, they are specific and they accelerate the rate of only one kind of chemical reaction of one type of specific compound, and their analysis is far more efficient manner than human-made catalysts. The proteins are controlled by inhibitors and activators that initiate or block reactions. All cells contain enzymes, which usually are different in number and composition, and also depends on the celltype; forexample, an average mammalian cell, is approximately one one-billionth (10−9) the size of a drop of water and generally contains about 3,000 enzymes.
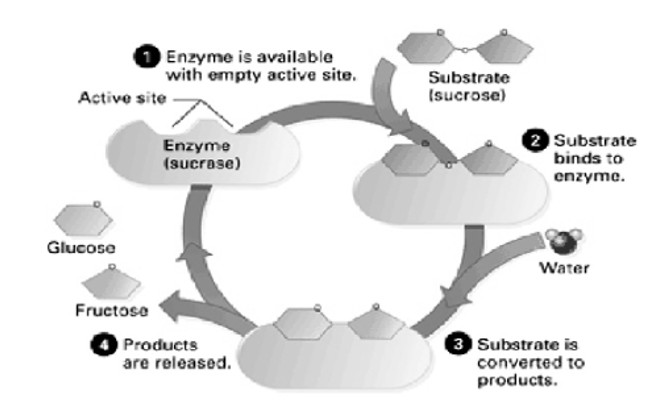
Shows the various steps involved during the process of enzyme action
Transporters
A transport protein (that is referred in many terms as a transmembrane pump, transporter, acid transport protein, escort protein, cation transport protein, or anion transport protein) is a protein whose main purpose is to move other materials within an organism. Transport proteins are vital for the growth and life of all living organisms.
Transport Protein Definition
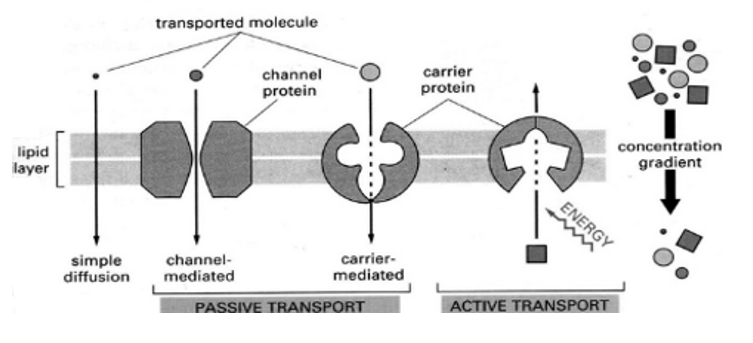
Transport proteins are proteins that mainly function to transport substances across biological membranes. The occurrence of proteins is found to be within the membrane itself, where they form a channel, or a carrying mechanism, wherein they allow their substrate to pass from one side to the other end.
The substances include sodium and potassium ions also sugars like glucose, messenger molecules, proteins and many more are transported by these proteins.
Transport proteins generally perform transport that is divided into two ways of transport: “facilitated diffusion,” where a transport protein creates an opening for a substance to diffuse down its concentration gradient; and “active transport,” where the cell expends energy in order to move a substance against its concentration gradient.
Function of Transport Protein
As we know certain important molecules, such as DNA, must be present inside the cell always; however other molecules such as ions, sugars, and proteins, can move in and out of the cell to function properly.
Each transport protein is designated to transport a specific substance when required. For example, some channel proteins, open only when they receive the correct signal, allowing the substances they transport to flow on demand. Active transporters, likewise, can often be “turned on and off” by messenger molecules.
Nerve impulses and cellular metabolism are possible by moving substances across membranes by transport proteins.
These proteins are so very important as the transport proteins, for example, the sodium-potassium gradient that allows our nerves to fire would not exist.
Types of Transport Proteins
Channels/Pores
As their name suggests, “channel” or “pore” proteins help in opening holes in the membrane of a cell.
These proteins are characterized by being open at the same time for both the intracellular and extracellular activities. In contrast, to carrier proteins which are only open to the inside or outside of a cell at any specific time.
Channels or pores are typically designed in such a way that only one specific substance can pass through.
For example, voltage-gated ion channels often use charged amino acids, that are present at specific distances, to attract their desired ion while all the others are repelled. The desired ion can then flow through the channel while other substances cannot.
A good example of transport proteins are the Voltage-gated ion channels are that act specifically. they are often seen in neurons, voltage-gated ion channels open in response to changes in a membrane’s electrochemical potential.
When closed, the voltage-gated channel does not allow any ions to pass through the cell membrane. But when open, it allows huge quantities of ions to pass through very quickly, allowing the cell to change its membrane potential rapidly and fire a nerve impulse.
Q3) Describe the function of proteins?
A3) Proteins play an important role in many crucial biological processes and functions. They are very versatile and have many different functions in the body, as listed below:
- They act as catalysts
- Transport other molecules
- They store other molecules
- Provide mechanical support
- Provide immune protection
- Generate movement
- Transmit nerve impulses
- Control cell growth and differentiation
The changes in the structure of a protein occurs when minute changes occur in their function or structure, the extent to which the structure of proteins has an impact on their function is shown by the effect of changes in them. Any change to a protein at any structural level, including slight changes in the folding and shape of the protein, may render it non-functional.
Q4) What are carrier Proteins?
A4) Carrier proteins are transport proteins that are only open to one side of the membrane at a particular time.
As they transport substances against the concentration gradient. They are often designed this way. If the membrane is open simultaneously to both sides it might allow these substances to simply flow back along their concentration gradient, putting an end to the carrier protein work.
To achieve their work, carrier proteins change their shapes and utilise the energy.
The sodium-potassium pump, for example, uses the energy of ATP to change its shape from being open to the intracellular solution, to being open to the extracellular solution. This allows it to collect ions inside the cell and release them outside of it, and then vice versa.
Other carrier proteins may use other energy sources, such as existing concentration gradients, to accomplish “secondary active transport.” This means that their transport is made possible through the cell expending energy, but the protein itself does not use ATP directly.
The carrier proteins often use the energy of one substance that “wants” to move down its concentration gradient in order to change its shape. The same shape change allows it to transport a substance that “doesn’t want” to move at the same time.
A good example is the sodium-glucose transport protein which uses the concentration gradient of sodium – originally created by the sodium-potassium pump – to move glucose against its concentration gradient.
We discuss the sodium-potassium pump and the sodium-glucose transport protein in detail below.
Q5) Describe Receptor proteins?
A5) A receptor is a protein which binds to a specific molecule. The molecule it binds is known as the ligand. A ligand may be any molecule, from inorganic minerals to organism -created proteins, hormones, and neurotransmitters. The ligand binds to the ligand-binding site on the receptor protein. When this binding happens, the receptor undergoes a conformational change. This change is shape slightly alters the protein’s function. From this, a number of things can happen. The conformational change in the receptor can cause the receptor to become an enzyme and actively combine or separate certain molecules.
The change can also cause a series of changes in related proteins, eventually transferring some sort of message to the cell. This message could be a metabolic regulation message, or it could be a sensory signal. The receptor has a certain capacity to hold onto the ligand, known as the binding affinity. Once this attraction wears out, the receptor will release the ligand, undergo a change to the original shape, and the message or signal will end. The speed of this turnover depends on the strength of the affinity between receptor and ligand.
Other molecules can also attach to the ligand-binding site on a receptor. These are called agonist molecules if they mimic the effect of the natural ligand. Many drugs, both prescription and illegal, are synthetic agonists to molecules like endorphins, which create feelings of satisfaction. However, these molecules often have a stronger affinity for the receptor than the natural ligand does. This means the agonist will stay attached to the receptor longer, which is why tolerances develop to certain drugs and painkillers. To get the same number of nerves firing when so many are already blocked by the drug requires a much higher dosage.
Still other molecules can act like antagonists, or molecules which block the ligand binding site on the receptor but do not allow the receptor to undergo a conformation change. This blocks a signal entirely. Some receptor antagonists include drugs which are used to wean people off of heroin and alcohol dependency. These act by making the use of the drug no longer pleasurable. Other antagonists include certain proteins in snake venom which mimic platelet binding proteins. The receptors which would normally connect platelets and prevent bleeding are therefore disabled. This can lead to internal bleeding and death. Pharmaceutical companies are interested in both agonists and antagonists for their potential to create effective medicines.
Types of Receptors
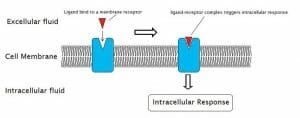
There are literally thousands of different types of receptors in the mammalian body. While there are far too many to start listing out, receptors do fall into some very broad categories of function. Many are used in “cellular signalling”, which is an enormously complex system of signals and responses mediated almost entirely by receptors and the ligands they receive. These include receptor proteins embedded in the cellular membrane which activate other sequences upon receiving a ligand, and the receptors found in the immune system which are structured to find intruding proteins and molecules. Below is the general model for cell signalling, which can take many different forms.
Q6) Explain proteins as structural elements?
A6) Structural proteins confer stiffness and rigidity to otherwise-fluid biological components. Most structural proteins are fibrous protein; for example, collagen and elastin are critical components of connective tissue such as cartilage, and keratin is found in hard or filamentous structures such as hair, nails, feathers, hooves, and some animal shells Some globular proteins can also play structural functions, for example, actinand tubulin are globular and soluble as monomers, but polymerizeto form long, stiff fibers that make up the cytoskeleton, which allows the cell to maintain its shape and size.
Other proteins that serve structural functions are motor proteins such as myosin, kinesin, and dynein, which are capable of generating mechanical forces. These proteins are crucial for cellular motility of single celled organisms and the sperm of many multicellular organisms which reproduce sexually. They also generate the forces exerted by contracting muscles and play essential roles in intracellular transport.
Q7) Define denaturation in proteins?
A7) Denaturation of proteins involves the disruption and possible destruction of both the secondary and tertiary structures. Since denaturation reactions are not strong enough to break the peptide bonds, the primary structure (sequence of amino acids) remains the same after a denaturation process.
Proteins are built of folded chains of compounds called amino acids. The process that causes a protein to lose its shape is known as denaturation. Denaturation is usually caused by external stress on the protein, such as solvents, inorganic salts, exposure to acids or bases, and by heat.
The way proteins change their structure in the presence of certain chemicals, acids or bases - protein denaturation - plays a key role in many important biological processes. And the way proteins interact with various simple molecules is essential to finding new drugs.
Q8) Define Collagen and its uses?
A8) Collagen is the most abundant protein in your body, accounting for about a third of its protein composition.
It’s one of the major building blocks of bones, skin, muscles, tendons, and ligaments. Collagen is also found in many other body parts, including blood vessels, corneas, and teeth. Collagen is the major insoluble fibrous protein in the extracellular matrix and in connective tissue. In fact, it is the single most abundant protein in the animal kingdom. There are at least 16 types of collagen, but 80 – 90 percent of the collagen in the body consists of types I, II, and III
Q9) Explain the hierarchy of Proteins?
A9)
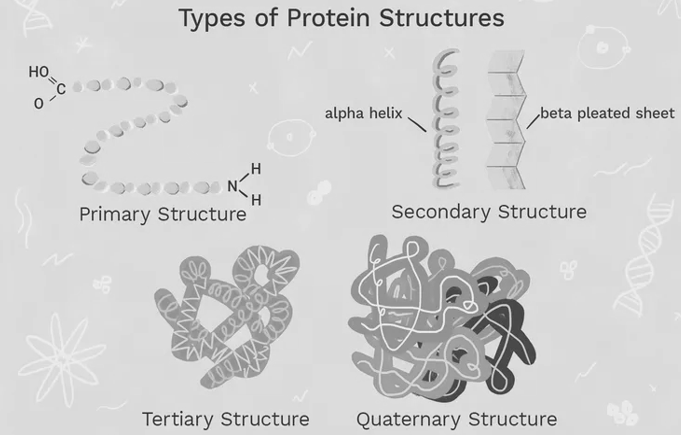
- In particular, Linderstorm -land (1952) first proposed that there was a hierarchy of protein structure with four levels: primary, secondary, tertiary, and quaternary. This hierarchy of protein is much easier as this structural classification is very convenient and anyone could start with this concept to teach the basic protein structure and this is probably familiar with this hierarchy as this structural classification is the easy and dependable. To revise: each level of the hierarchy is 'held together' by characteristic interactions and forces
- Higher levels of structure in the hierarchy are composed of the structural entities (also called elements) of the lower levels
Primary protein structure
Proteins are made up of a long chain of amino acids. In the human body they are around 20amino acids that are commonly seen with a limited number of amino acid monomers. The proteins can be arranged in enormous number of ways to the function and alter the three-dimensional structure of the protein. The primary structure consists of the simple sequencing of the protein.
Secondary protein structure
The secondary protein structure depends on the local interactions that occurs between parts of a protein chain, this can affect the folding and three-dimensional shape of the protein. There are two main things that can alter the secondary structure:
- α-helix: sN-H groups in the backbone form a hydrogen bond with the C=O group of the amino acid 4 residues earlier in the helix.
- β-pleated sheet: In this structure the N-H groups that lies in the backbone of one strand form hydrogen bonds with C=O groups in the backbone of a fully extended strand next to it.
Proteins can be linked with several functional groups such as alcohols, carboxamines, carboxylic acids, thioesters, thiols, and other basic groups linked to each protein. These functional groups also affect the folding of the proteins and, thereby, its function in the body.
Tertiary structure
The tertiary structure of proteins, the proteins three-dimensional shape is taken into consideration, after the secondary interactions. These include the influence of non-polar, polar, acidic, and basic R groups that exist on the protein.
Quaternary protein
The quaternary protein structure in this case refers to the arrangement and orientation of subunits in proteins that possess multi-subunits. This is only relevant for proteins with multiple polypeptide chains.
According to the sequence of amino acids in a polymer theProteins subsequently fold up into specific shapes, and the protein function is directly related to the resulting 3D structure.
Proteins are also known to interact with each other or other macromolecules present in the body to create complex structures. In these assemblies, proteins can develop certain functions that would not have been possible as an individual protein, such as carrying out DNA replication and the transmission of cell signals.
The nature of proteins is also showing a lot of variation. For example, some are quite rigid, whereas others are somewhat flexible. These characteristics in proteins also makes them capable for the function of the protein. For example, more rigid proteins can play a role in the structure of the connective tissues or cytoskeleton. On the other hand, those with some flexibility may act as hinges, springs, or levers to assist in the function of other proteins.
Q10) What does the reductionist level of analyses of molecules?
A10) Reductionism, by its nature, cannot be detailed or compared with the complexity of biological systems, the properties of these biological systems cannot be explained or predicted by studying their individual components. Subsequently these biological systems that are studied by scientists, now understand that the individual components of biological systems such as molecular pathways never work alone—they function in highly structured and integrated biological networks.
The changing properties and dynamics of these biological systems occur due to health and disease. Thus, a more holistic approach is required to gain a true understanding of health and disease, including the integrated analysis at broadly desperate levels, from molecular to organismal, from genetic to environmental. When scientists unravel this complexity, decipher these dynamic network interactions, and develop predictive models, then an understanding to life opens up.
All systems are working in synergy to analyse the multiplescientific developments and to advance systems biology. Firstly, new tools and technologies in today’s world is enabling analysis of complex networks of the changing dynamics that define healthy and disease states. Secondly, the enormous amount of data for storing and distributing has become easier. Last but not least, an expanded ecosystem of scientists is breaking down research silos and working together across disciplines.