Unit - 5
Alloying of Steel
Q1) Explain Various Kinds of Steel with their Properties.
A1)
- High Speed Steel-
- High speed steel is widely used for cutting of metals where hardness must be retained at elevated temperatures.
- These steels are obtained by alloying 18% tungsten, 4% chromium and 1% vanadium with a carbon content of 0.6 to 0.7%. This alloy is termed as 18:4:1 while an increase of vanadium to 2% produces 18:4:2 steels.
- In addition to heat resistance high speed steels have the desirable properties pf high hardness, high compressive strength and outstanding wear resistance.
- Uses: This steel is used for high-speed cutting tools.
2. Heat Resisting Steel-
- Steels which must be resistance to creep at high temperature must contain molybdenum, silicon and chromium impart resistance to oxidation and scaling.
- Steels which are satisfactory up to about
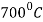 operating temperature are: C= 0.15%, Si = 0.5-20%, Mn = 0.5% maximum, Ni = none, Cr = 1 to 6%, Mo = 0.5%.
operating temperature are: C= 0.15%, Si = 0.5-20%, Mn = 0.5% maximum, Ni = none, Cr = 1 to 6%, Mo = 0.5%. - Uses: These are used in the valves of internal combustion engines in rolled or in forged condition. For higher temperature up to
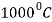 , steels containing up to 22% nickel and 26% chromium are used.
, steels containing up to 22% nickel and 26% chromium are used.
3. Silicon Steel-
- Improves the electrical properties of steel.
- Silicon imparts fatigue strength and resistance to steel.
- Steel containing silicon has very low magnetic hysteresis.
- Uses: Steel with Mn = 1%, Si = 2%, C = 0.4 to 0.6% has very high elastic limit and is used for springs.
- 13% silicon content steel has a very high corrosion resistance so it is used in the chemical industries.
4. Stainless Steel-
- Plain Chromium and High Chromium Low Nickel Steel:
Out of this group the former has C= 0.8%. Cr = 12 to 20% and latter has C = 0.1 to 0.2%, Cr = 12 to 20% and Ni = 2%.
These steels can be heat – treated.
Uses: For dies, valves and cutlery.
b. Chromium- Nickel Steel:
These steels are non-magnetic and cannot be hardened.
They have varieties due to varying contents of chromium and nickel respectively e.g., small quantity of copper, tungsten and molybdenum is also added to these steels.
They have high resistance to corrosion and may be cold or hot worked, pressed welded, brazed or soldered.
These steels are poor conductors of heat and electricity.
Uses: It is used in making utensils.
Q2) Write a note on various types of stainless steel with their properties.
A2) Stainless steel is divided into three different classes on the basis of the phase constituent of the microstructure. These are;
- Martensite stainless steel
- Ferrite stainless steel
- Austenitic stainless steel
- Properties of Martensite stainless steel:
- Martensitic stainless steel contains 12 to 17% Cr with sufficient carbon.
- It is capable of being heat treated in such a way that the martensite is the prime microconstituent.
- It is hardenable and magnetic.
2. Properties of Ferrite stainless steel:
- Ferritic stainless steel contains 12 to 30% Cr with low carbon.
- It is mostly composition of a ferrite (BCC) phase.
- It is soft, ductile, corrosion resistant and magnetic.
3. Properties of Austenitic stainless steel:
- Austenitic stainless steel contains 16 to 25% Cr and 7 to 20% Ni.
- Its crystal structure contains FCC structure. Due to FCC structure it is highly formable.
- It is non-magnetic.
Q3) What is Maraging Steel?
A3) Maraging steels are carbon free iron-nickel alloys with additions of cobalt, molybdenum, titanium and aluminium. Maraging steels are characterized with superior strength combined with excellent toughness properties and weldability. The term ‘maraging’ refers to the strengthening mechanisms and was coined from a combination of martensite and age-hardening; ‘mar’ refers to martensite and ‘aging’ refers to the age hardening heat treatment. Maraging steel is forged by a martensitic transformation followed by subsequent age-hardening.
These steels are a special class of low-carbon ultra-high-strength steels that derive their strength not from carbon, but from precipitation of intermetallic compounds. The principal alloying element is 15 to 25 wt.% nickel (Ni). Secondary alloying elements, which include cobalt (Co), molybdenum (Mo), aluminium (Al), and titanium (Ti), are added to produce inter metallic precipitates.
Q4) What is Cast iron? Define Gray Cast iron and white cast iron with their properties.
A4) Cast iron is very useful engineering material. It contains more carbon percentage (approx., 2%<C<4.5%).
When pig iron is melted with coke and limestone in cupola furnace, it produces cast iron.
Coke acts as fuel and limestone acts as flux.
Fluxes are used to separate the impurities from the pig iron.
Properties:
- It has a good hardness due to presence of high carbon percentage.
- Its compressive strength is very good.
- It has good machinability.
- It has very good rigidity.
- Gray Cast Iron:
Composition-
Element | Gray Iron (%) |
Carbon | 2.5 – 4% |
Silicon | 1 – 3% |
Manganese | 0.25 – 1% |
Sulphur | 0.02 – 0.25% |
Phosphorous | 0.05 – 1% |
Properties:
- Tensile strength varies between 1500 and 4000 kg/
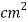 .
. - Hardness between 155 and 320 BHN, and
- Compressive strength is 3-4 times the tensile strength.
2. White Cast Iron:
White cast iron is a type of carbon-iron alloy that contains carbon content greater than 2% in the form of cementite. The name white cast is derived from its white surface, which is caused by carbide impurities that allows cracks throughout the metal.
Properties-
- White cast iron has high compressive strength and wears resistance.
Q5) Differentiate between malleable and Speroidal cast iron with their properties.
A5)
- Malleable Cast Iron:
Composition:
Element | Malleable iron (%) |
Carbon | 2 – 2.60% |
Silicon | 1.10 – 1.60% |
Manganese | 0.20 – 1% |
Sulphur | 0.04 – 0.18% |
Phosphorous | 0.18% (max) |
Properties-
- It is the ductile iron.
- Exhibits better toughness fracture properties in low temperature.
- Easy castability.
2. Spheroidal Cast Iron:
Spheroidal Graphite Cast Iron 600/3, also known as Ductile Cast Iron.
Composition:
Element | Ductile Cast Iron (%) |
Carbon | 3.2 – 4.5% |
Silicon | 1 – 3% |
Manganese | 0.1 – 1% |
Sulphur | 0.3% (max) |
Phosphorous | 0.1% (max) |
Properties:
- This kind of cast iron has got very high fluidity, castability, strength, toughness, wear resistance, pressure tightness, weldability and machinability.
- Because of its excellent casting quality, it is suited for both intricate casting as well as big size castings.
Q6) Define copper with its physical properties.
A6) Copper-
- Copper is most widely used non-ferrous metal in industry.
- It has a very good mechanical properties as well as physical properties.
- It is soft, malleable, and good conductor of electricity. Due to formation of copper oxide layer, it becomes a highly corrosion resistant materials.
Physical properties:
Structure | Crystalline (FCC) |
Colour | Reddish- Brown |
Density | 8.96* 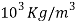 |
Melting Point | 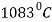 |
Boiling Point | 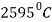 |
Q7) Elaborate some of the alloys of copper.
A7) Major Alloys of Copper are:
- Brass
- Bronze
- Cupro-nickel, and many more.
- Brass (Cu-Zn Alloys):
Brass is an alloy of copper and zinc. In brasses Cu and Zn proportions vary and gives rise of various types of brasses and improve its properties.
Good property brasses can be achieved by adding a small amount of alloying elements. As addition of lead alloy by 1 to 1.5% improves its strength and machining property but reduces its thermal and electrical conductivity.
Th e importance of brass is its corrosion resistant property towards exposure of environment. Thus, brass is used for electroplating of metals. They have good fabrication property.
Classification:
Family | Principal alloying element |
Yello Brass | Zn |
Leaded Brass | Pb |
Nickel Brass | Ni |
Various types of Brasses:
- Gliding Metal-
Its composition is 85%Cu + 15%Zn.
- Cartridge Brass-
Its composition is 70%Cu + 30%Zn.
- 63/37 Brass-
Its composition is 63%Cu + 37%Zn
- Yellow Brass (Muntz Metal)-
Its composition is 60%Cu + 40%Zn
2. Bronze (Cu-Tin Alloy):
To Bronzes are the alloys of copper and tin. The composition of bronzes generally 75 to 95%copper and 5 to 25% tin.
Bronzes are hard and resistant to surface wear. Corrosion resistant properties of bronzes are comparatively better than brasses.
Classification:
Family | Principal alloying element |
Phosphor bronze | Tin (Sn) |
Aluminium Bronze | Aluminium (Al) |
Silicon Bronze | Silicon (Si) |
3. Phosphor Bronze-
Properties:
- Bronze containing phosphorous are called phosphor bronze.
- Due to presence of phosphorous its strength and ductility increases.
- It has good wearing quality and corrosion resistant property to salt water.
Composition:
87-90% Cu, 9-10% Sn and 1-3% P.
4. Silicon Bronze-
Properties:
- Silicon bronze is a high strength engineering alloy.
- It can be easily cast, rolled, forged, stamped and pressed in both hot and cold working processes.
Composition:
96% Cu+ 3% Si + 1% Mg or Zn.
5. Beryllium Bronze:
- It has high wear resistance property (approximately 5 times that of Ph. Bronze) so it may be used as a bearing metal.
- It also has lubricating property which improves its quality as bearing metal. It has high fatigue limit and good yield strength.
- It has excellent corrosion resistant property towards hot and cold environment.
Composition:
97.7% Cu and 2.3% beryllium.
6. Cupro-Nickel alloy:
It refers to a mixture consisting of copper and other elements like nickel and various strengthening components like manganese and iron among other.
Addition of Nickel on to Copper and other elements merely strengthens this material, makes it ductile and shiny.All these are characteristics of a high-quality metallic material.
Copper-nickel alloy pipe can withstand extremely high temperatures. In fact, this is one of its utmost outstanding elements which makes it popular in several applications. This material is known for enduring temperatures that even exceed 760 degrees Celsius.
Q8) What is Aluminium? Explain with its physical properties.
A8)
- Aluminium is produced by electrical processes from its ore alumina which is prepared from its mineral bauxite.
- It has very good mechanical and physical properties.
- Aluminium has good electrical conductivity and it is highly resistance to corrosion and non- toxic in nature. It has low density and weight.
Structure | Crystalline (FCC) |
Colour | White metal |
Density | 2.7* 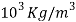 |
Melting Point | 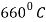 |
Boiling Point | 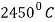 |
Q9) Define Alloys of Aluminium with their properties.
A9) Cu-Al Alloy (Y-alloys):
- It is an alloy of copper – aluminium. Copper increases its strength and machinability property.
- A very small quantity upto 0.6% of each Si, Mg and Fe is also present in Y-alloy. It has better strength than duralumin at high temperature.
- Magnalium:
- Magnalium is produced by melting the aluminium with 2 to 10% of magnesium in absence of air (or in vacuum) followed by cooling in vacuum or under a pressure between 100 to 200 atm.
- It also contains a very small amount of copper (approx.,1.75%).
2. Mg-Alloy:
- Magnesium alloys are mixtures of magnesium with other metals (called an alloy), often aluminium, zinc, manganese, silicon, copper, rare earths and zirconium. Magnesium is the lightest structural metal.
- The advantage of magnesium alloys to be used in such applications is their light weight, high strength-to-weight ratio, high stiffness-to-weight ratio, castability, machinability, and great damping.
3. Nickel-based Superalloys:
- Nickel-based superalloys possess good combinations of high-temperature mechanical properties and oxidation resistance up to approximately 550°C.
- High temperature heat-resistance alloys, which can retain high strengths at elevated temperatures.
- Alloying increases the strength and temperature capability but reduces the processability.
PROPERTIES:
- Heat resistant and high strength at high temperature (760-980oC).
- Good corrosion resistance.
- Good oxidation resistance.
- High toughness and ductility
- Excellent cryogenic temperature properties.
Q10) Mention the Nickel and Titanium based superalloys with their properties and uses.
A10) There are three types of Ni-base superalloys:
• Nickel base,
• Nickle-lron base
• Cobalt base.
The alloys contain high Cr with Ti, Al to from precipitates and additions of Mo, Co, Nb, Zr, B,Fe.
Titanium Alloy:
Titanium alloys are alloys that contains traces of aluminum, molybdenum, vanadium, niobium, tantalum, zirconium, manganese, iron, chromium, cobalt, nickel, and copper.
Such alloys have very high tensile strength and toughness (even at extreme temperatures). They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures.
Titanium alloys possess high specific properties, have a good fatigue strength/tensile strength ratio with a distinct fatigue limit, and some retain considerable strength at temperatures up to 400–500°C.
Uses:
Titanium has outstanding corrosion resistance to seawater, and thus is used in propeller shafts, rigging and other parts of boats that are exposed to seawater. Titanium and its alloys are used in airplanes, missiles, and rockets where strength, low weight, and resistance to high temperatures are important.