Unit 1
Fundamentals
Q1. Explain the term System and different types of systems.
A1. System
Thermodynamic system is the region which is under observation for thermodynamic changes.
There are 3 types of systems thermodynamics
- Isolated systems,
- Closed system, and
- Open system
An isolated system cannot exchange either mass or energy with the surroundings.
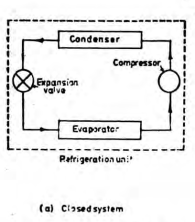
A closed system exchanges energy with the surrounding but mass transfer is not possible.
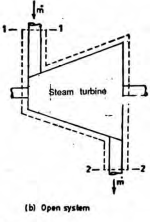
An open system can exchange both mass and energy with the surroundings.
Q2. Define Path functions.
A2. The properties that are expressed at a particular state of the system and are not at all dependent on the path followed by the system are called path functions. Examples are pressure, temperature, volume, etc.
Exact differentials are the derivatives of some other functions as the name suggests.
These are used to express change in point functions. Examples are pressure change dP, volume change dV, etc.
Q3. Explain the term displacement work in thermodynamics.
A3. Displacement Work:
Consider a piston sliding inside the cylinder as shown in the figure.
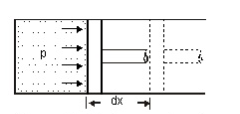
The pressure is being exerted on the piston by the expanding fluid which causes the piston to displace towards right.
Force acting on the piston, F = pressure, p x area, A
= pA
Work done in displacement = Force x displacement
W = pA x dx
= pdV
This work done is always dependent and changes according to the path followed by the system.
Q4. Derive an expression for work done during a simple Isobaric process.
A4.
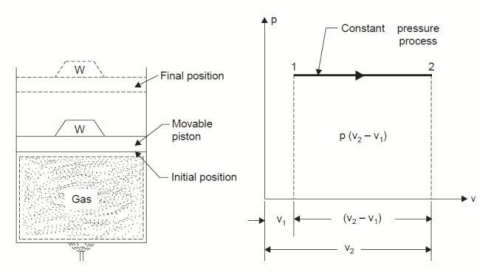
Considering unit mass of working substance and applying first law of thermodynamics to the process,
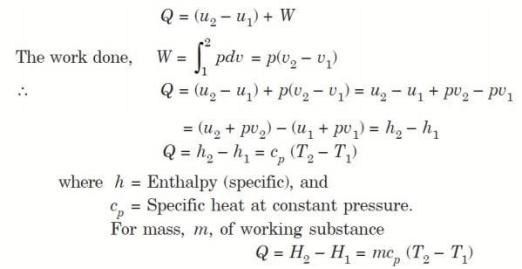
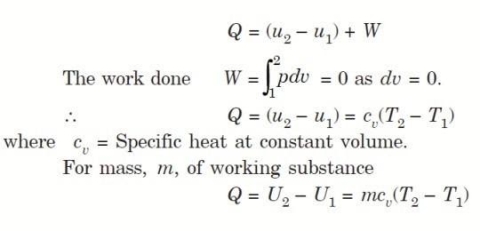
Q5. Derive an expression for work done during a simple Isochoric process.
A5.
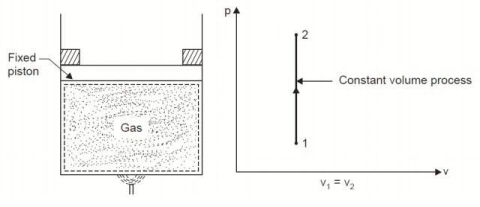
Considering mass of the working substance unity and applying first law of thermodynamics to the process,
Q6. Derive an expression for work done during a simple Isothermal process.
A6.
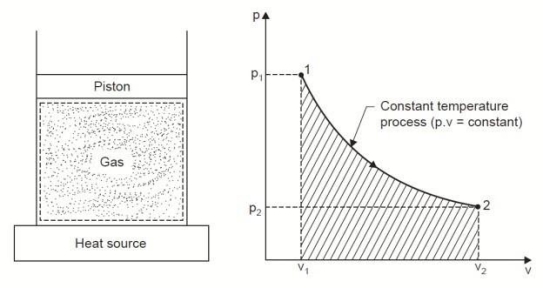 Considering unit mass of working substance and applying first law to the process,
Considering unit mass of working substance and applying first law to the process,
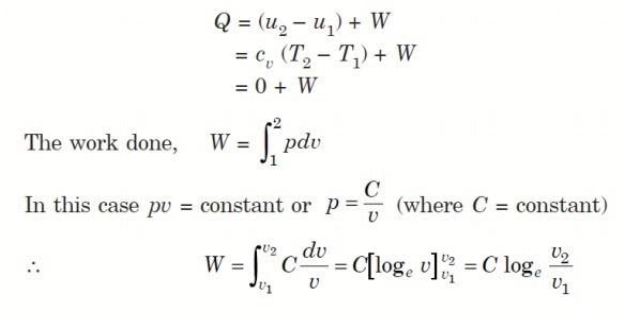
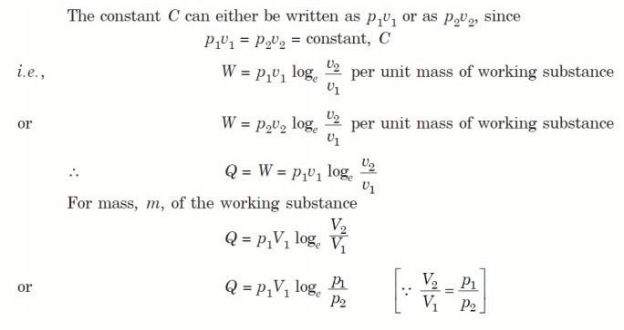
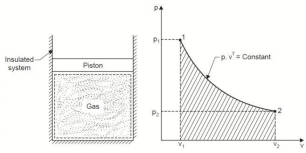
Q7. Derive an expression for work done during a reversible adiabatic process.
A7. An adiabatic process is one in which no heat is transferred to or from the fluid during the process. Such a process can be reversible or irreversible.
Considering unit mass of working substance and applying first law to the process
Q = (u2 – u1) + W
O = (u2 – u1) + W
or W = (u1 – u2) for any adiabatic process
Above equation is true for an adiabatic process whether process is reversible or not.

Q8. A certain gas has Cp = 0.913 and Cv = 0.653 kJ/kg K. Find the molecular weight and the gas constant R of the gas.
A8. Gas constant = R = Cp – Cv = 0.913 - 0.653
= 0.26 kJ/kg-K
If molecular weight of the gas is M, then
R0 = MR
M = R0 / R
= 8.3143 / 0.26
= 31.98 kJ/kg-mole
Q9. One kg of air in a closed system, initially at 5°C and occupying 0.3m3 volume, undergoes a constant pressure heating process to 100°C. There is no work other than pdv work. Find the work done during the process.
A9. 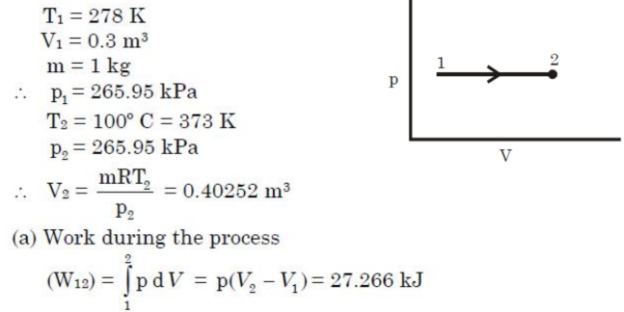
Q10. Explain the term Electrical Work.
A10. Electrical Work:
Electrical work is defined as the work required to move an unit charge once around the complete circuit.
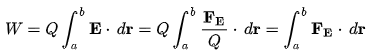
Here, Q = charge on the particle.
E = strength of electric field,
FE = Coulomb force
r = displacement of particle under the action of the Coulomb force.
Q11. Explain shaft work and spring work.
A11. Spring Work:
When a force is applied on spring, it compresses or expands, depending on the direction of the applied force.
This displacement causes some work to be done on the spring.
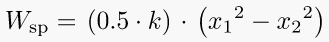
Where, k = stiffness constant of the spring,
x1 = initial distance of the spring from datum
x2 = final distance of the spring from the datum
Shaft Work:
When a shaft is rotated by some external force, work is done on the shaft.
It is given by,
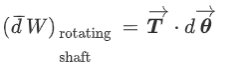
Where, T= torque acting on the shaft
dθ = angular displacement in radians.