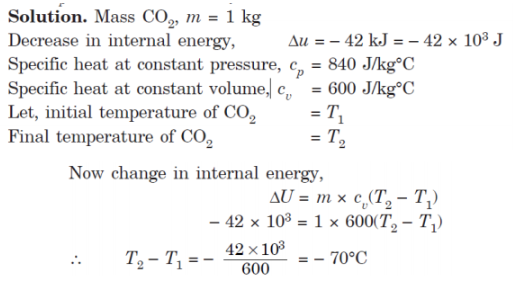Unit – 2
Temperature
Q1. Define Temperature.
A1. Temperature:
The temperature of a substance is defined as measure of hotness or degree of coldness of a body. We know that energy flows from higher level to lower level in the universe. So heat flows from higher temperature body to lower temperature body.
Temperature is not a measure of quantity of energy possessed by the body but rather, it indicates level of internal energy possessed by the body
The absolute temperature is the temperature measured above the point of absolute zero. Absolute temperatures are expressed by the capital letter T.
Q2. Explain the Zeroth law of thermodynamics.
A2. Zeroth Law of Thermodynamics:
If Body 1 is in thermal equilibrium with Body 3 and Body 2 is in thermal equilibrium with Body 3, it implies that Body 1 is also in thermal equilibrium with Body 2.
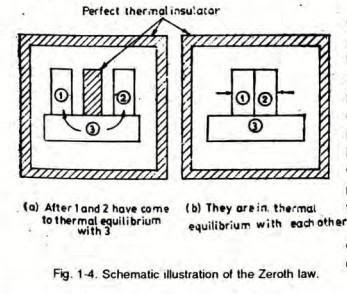
Thermal equilibrium is a state where no heat is transferred between two bodies in contact or between system and surrounding.
Q3. What are various degree-based temperature scales that are used in measurement of temperature?
A3.
- Freezing point of water: 32° F
- Boiling point of water: 212° F
- Coldest possible temperature: -459.67° F (absolute zero)
2. Celsius Scale: Most commonly used system and scale in the world. Also termed as Centigrade scale since it divides the difference in freezing and boiling point of water into 100 divisions. It can also express negative temperatures. Following are the important points on this scale
Q4. What are various derived scales from degree-based temperature scales that are used in measurement of temperature?
A4.
- Freezing point of water: 273.15 K
- Boiling point of water: 373.15 K
- Coldest possible temperature: 0 K (absolute zero)
- You can convert from Celsius to Kelvin by adding 273.15 to Celsius temperature.
2. Rankine Scale: It has been adapted from the Fahrenheit scale. It was designed to set the absolute zero temperature at 0. Following are the important points on this scale
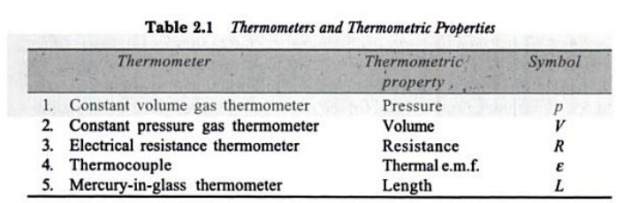
Q5. Define thermometers. What are different types of thermometers with their thermometric properties?
A5. Thermometers:
A thermometer is a device that is used to measure the temperature of a system.
Thermometers are based on the principle that some physical property of a system changes as the system’s temperature changes.
Mercury bulb thermometer is the most commonly used, since mercury has a very high co-efficient of thermal expansion.
Various types of thermometers that are used are tabulated below with their principle of operation.
Q6. Define Heat.
A6. Heat (Q):
Heat is a form of energy that flows between bodies or parts of body due to difference in temperature.
Heat will always flow from body at higher temperature to lower temperature.
It is measured in Joules or Calories.
In thermodynamic system, heat produced by the system or heat rejected is taken as negative and heat absorbed by the system is taken as positive.
The total amount of heat transferred in a process is shown in the graph below.
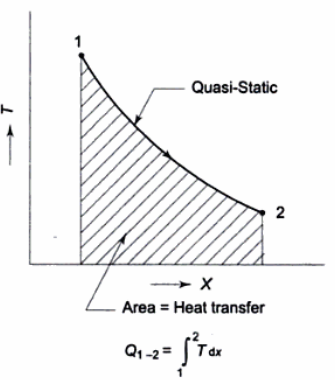
It is the area under the curve 1-2 and can be found by integration as given above.
Q7. Explain the application of First law of thermodynamics for cyclic and non-cyclic process.
A7. First law of thermodynamics for cyclic and non-cyclic process:
There are different laws of thermodynamics while the first law of thermodynamics deals with the processes that are cyclic and non-cyclic.
For cyclic process:
For Non-cyclic process:
Q8. Explain the Concept of Energy.
A8. Concept of Total Energy:
The total energy content of a system is the sum of Kinetic energy, Potential energy and the internal energy contained in the system.
E = KE + PE + IE
E = ½ mv2 + mgh + U
In most of the systems, KE and PE are negligible as compared to Internal Energy of the system which is due to vibrational motion of the atoms.
Hence, in general, E = U
Then Q = U + W
In differential form, dQ = dU + dW
In integral form,
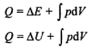
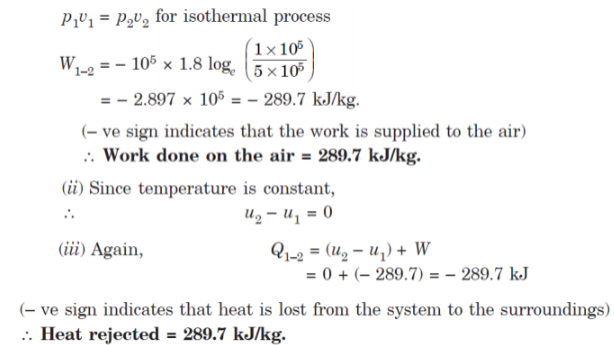
Q9. Air enters a compressor at 105 Pa and 25°C having volume of 1.8 m3/kg and is compressed to 5 × 105 Pa isothermally.
Determine:
(i) Work done;
(ii) Change in internal energy; and
(iii) Heat transferred.
A9.
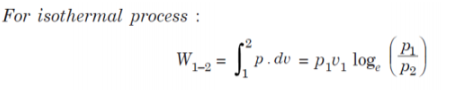
Q10. 1 kg of gaseous CO2 contained in a closed system undergoes a reversible process at constant pressure. During this process 42 kJ of internal energy is decreased. Determine the work done during the process. Take Cp = 840 J/kg°C and Cv = 600 J/kg°C.
A10.