SYSTEMATIC CHEMISTRY OF THE ELEMENTS
Q1). How is Hydrogen different from Halides?
A1). Hydrogen is the first element of the periodic table. Its electronic configuration is 1. Due to the presence of only one electron in its K shell, hydrogen exhibits a dual behaviour, i.e., it resembles both alkali metals and halogens. As a result, it is placed at the top of the alkali metals in group 1 and also, along with halogens in group 17, since, just like halogens, it also requires one electron to have the configuration of the nearest noble gas element.
To attain the nearest noble gas configuration, both the halogens and the hydrogen need one electron to complete their octet and duplet, therefore hydrogen can gain one electron to produce a uni-negative ion. Hydrogen has an electrogene configuration of 1 and that of Fluorine is 2,7, both of them gain one electron each to attain the noble gas configuration of helium and neon, respectively. Like halogens, it forms a diatomic molecule and several covalent compounds.
2Na + Cl2 → 2NaCl
H2 + Cl2 → 2HCl
Similarities to Halogens
H + e‾ → H‾
Cl + e‾ → Cl‾
2Na + H2 → 2NaH
Ca + H2 → CaH2
Differences with Halogens
Q2) What the hydrogen Isotopes?
A2) The three naturally occurring hydrogen isotopes are:
1H (protium), 2H (deuterium), and 3H (tritium). The other highly unstable ones are (4H to 7H) are processed in the lab, and do not occur naturally.
Of the naturally occurring isotopes, the most stable hydrogen radio isotope is tritium with a half-life of 12.32 years. The other heavier isotopes have a half-life less than a zeptosecond (10-21 sec). Of these, 5H is the most stable, and the least stable isotope is 7H.
Protium
1H is the very common hydrogen isotope and found in abundance in nature with almost more than 99.98%. The nucleus of the protium contains of only a single proton (atomic number = mass number = 1) and neutrons are absent in the nucleus. And its mass is 1.007825 amu. Hydrogen generally occurrence is diatomic in the form of hydrogen gas H2, hydrogen also combines with other atoms in the compounds. The occurrence of monoatomic hydrogen is rare. The H–H bond is one of the strongest bonds in nature, with a bond dissociation enthalpy of 435.88 kJ/mol at 298 K. therefore, H2 dissociates to only a minor extent until higher temperatures are reached. At 3000K, the degree of dissociation is only 7.85%. Hydrogen atoms are so reactive that they combine with almost all elements.
Deuterium
The other stable isotope of hydrogen is 2H, or deuterium (D), the natural abundance is around 156.25ppm in the oceans and around 0.0156% of hydrogen present on earth. The nucleus of deuterium is called as deuteron, consisting of one neutron and one proton (mass number = 2), due to the presence of neutron in the nucleus deuterium is twice the mass of protium (deuterium has a mass of 2.014102 amu, compared to the mean hydrogen atomic mass of 1.007947 amu). Deuterium occurs in trace amounts naturally as deuterium gas, written 2H2 or D2, but is most commonly found in the universe bonded with a protium 1H atom, forming a gas called hydrogen deuteride (HD or 1H2H).
When chemical aspects are noticed deuterium performs similar to protium, but they differ in bond energy and length of compounds of heavy hydrogen isotopes that are bigger than the isotopic differences than any other element. The bonds of deuterium and tritium are much stronger than protium, some of these differences are significant in biological reactions. Deuterium replaces the normal hydrogen in water molecules to form (D2O), which is about 10.6% denser than normal water. Heavy water is slightly toxic in eukaryotic animals, with 25% substitution of the body water causing cell division problems and sterility, and 50% substitution causing death by cytotoxic syndrome (bone marrow failure and gastrointestinal lining failure).
Is in NMR, as it requires compounds of interest to be dissolved in solution, the solution signal should not register in the analysis. As NMR analyses the nuclear spins of hydrogen atoms, the different nuclear spin property of deuterium is not ‘seen’ by the NMR instrument, making deuterated solvents highly desirable due to the lack of solvent-signal interference.
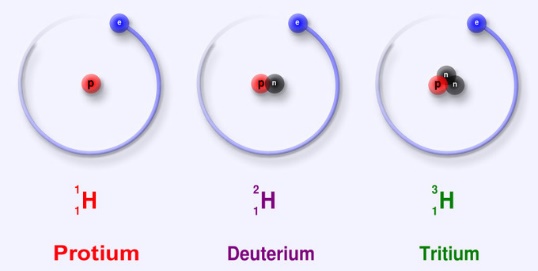
Fig :1Isotopes of Hydrogen The three naturally occurring isotopes of hydrogen.
Tritium
3H is known as tritium and contains one proton and two neutrons in its nucleus (mass number = 3). they are radioactive in nature that decay into helium-3 through beta-decay followed by the release of 18.6 keV of energy. It has a half-life of 12.32 years. Tritium occurs rarely on earth; trace amounts may be found when the cosmic rays interact with the atmosphere.
Q3) Define Para and Ortho Hydrogen?
A3) Ortho and Para Hydrogen
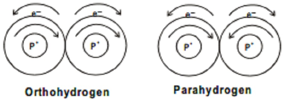
Fig 2: the Spins that occur in para and Ortho Hydrogen
The nucleus of the hydrogen atom spins about an axis like a top. When two hydrogen atoms combine, they form molecular hydrogen. Thus, depending on the direction of the two protons in the nucleus the following two types of hydrogen molecules are known.
When two hydrogens combine, molecular oxygen is formed, the nucleus of the hydrogen spin about their axis like a top.
Therefore, depending on the direction of the protos present in the nucleus. Two types of hydrogen are known to be formed. The two protons of the nuclei of both H-atoms, when they spin in the same direction, they are called as Ortho hydrogen, and when the protons of the nuclei of both H-atoms spin in opposite direction, it is called as parahydrogen.
The ordinary hydrogen contains 75% ortho and 25% para hydrogen at room temperature, but as temperature is lowered, the equilibrium shifts in favour of para form. At 25K There is 99% para and 1% ortho hydrogen. The change in the proportion of the two forms of hydrogen requires a catalyst such as platinum or atomic hydrogen or silent electric discharge.
The para hydrogen form was prepared originally made by absorbing ordinary hydrogen in activated charcoal present in a quartz vessel kept at a temperature of 20k. The charcoal absorbs almost pure para hydrogen. By this method, pure para hydrogen can be isolated.
Conversion of para into ortho hydrogen
Ortho hydrogen is more stable than para hydrogen. The para form is transformed into ortho form by the following methods.
1. By treatment with catalysts like platinum or iron
2. By passing an electric discharge
3. By heating to 800oC or more.
4. By mixing with paramagnetic molecules like O2, NO, NO2.
5. By mixing with nascent hydrogen or atomic hydrogen.
Properties: Ortho and para hydrogen are similar in chemical properties but differ in some of the physical properties.
(i) Melting point of para hydrogen is 13.83K while that of ordinary hydrogen is 13.95 K.
(ii) Boiling point of para hydrogen 20.26K while that of ordinary hydrogen is 20.39K.
(iii) Compared to the ordinary liquid hydrogen, the vapour pressure of liquid para hydrogen.
(iv) the magnetic moment of para hydrogen is twice than that of proton, where as that of para hydrogen is zero since the spins neutralise with each other
(v) Internal molecular energy is lower in parahydrogen than the ortho form.
.Q4) How are the Ionic Hydrides different from Covalent Hydrides?
A4) Ionic hydrides are formed when a molecule of hydrogen reacts with highly electropositive s-block elements (Alkali Metals and Alkaline Earth Metals). the ionic hydrides in solid state are non-conducting, crystalline and non-volatile, in liquid state they conduct electricity. Ionic hydrides on electrolysis liberate hydrogen gas at the anode. Saline or ionic hydrides does not dissolve in conventional solvents and they are mostly used as bases or reducing reagents in organic synthesis.
Example of Ionic Hydrides: Nah, KH, CaH2, etc. These contain hydrogen as the negatively charged (H–) ion.
Covalent hydrides are formed when hydrogen reacts with other similar electronegative elements like Si, C, etc. The most common examples are CH4 and NH3. In general, compounds that are formed when hydrogen is reacted with non-metals are called covalent hydrides. The compound shares a covalent bond and are either volatile or non-volatile compounds. Covalent hydrides are also either liquids or gases.
Example of Covalent Hydrides: SiH4 (silane)
Q5) Write a note on Hydrogen ion?
A5)A hydrogen ion is the nucleus of a hydrogen atom that has been separated from its electron. A proton is a particle with a unit positive electric charge that makes up the hydrogen nucleus. As a result, the isolated hydrogen ion, denoted by the symbol H+, is commonly used to describe a proton. So, the above-given definition helps to understand what is H+ ion and hydrogen ion formula.
The isolated hydrogen (H+ Ion) can only exist in a nearly particle-free space (high vacuum) and in the gaseous state siince the bare nucleus can easily combine with other particles (electrons, atoms, and molecules). The name hydrogen ion is commonly used to refer to the hydrogen ion found in water solutions, where it occurs as the combined molecule H+, H2O.
Hydrogen ion formula in Aqueous solution
The hydronium or oxonium ion is represented by the formula H+H2O, which is also written as H2O, which is also written as H3O+.The amount of hydrogen ion in a water solution is used to determine a substance’s acidity; the higher the hydrogen ion concentration, the more acidic the solution and the lower the pH.
Hydrogen Ion Concentration
Acidity or alkalinity is determined by the hydrogen ion concentration.
Let’s take the case of water. H2O is the formula for water, as you already know. The majority of water molecules are in a highly stable shape known as H2O. A small percentage of such compounds, however, have broken down into hydrogen ions (H+) and hydroxide ions (OH-).
In fact, the pH of water is determined by the balance of hydrogen and hydroxide ions.
The solution is acidic when the hydrogen ions outnumber the hydroxide ions. If the situation is reversed, the solution is alkaline. For any solution, the following relationship between the densities of hydrogen ions (H+) and hydroxide ions
(OH-) is observed if the temperatures do not change: (H+) (OH-) is observed if the temperature does not change: (H+) (OH-)=Kw=10-14(=fixed) at 25ºC
(Kw is called the ion product of water).
In pure water or neutral solution:
[H+] = [ OH- ]
[H+] = [ OH- ] = √ (Kw) = √ 10 -14 = 10 -7
If the value of either [H+] or [ OH-] is known the value of the other can be determined.
Thus, pH is determined by hydrogen concentration.
pH = - log 10 [H+]
Uses of hydrogen ion
In photosynthesis, hydrogen ions drive ATP synthase. As the hydrogen ions are forced through the membrane, a high concentration occurs within the thylakoid membrane and a low concentration occurs in the cytoplasm, Osmosis on the hand causes H+ ions to force its way out of the membrane through ATP synthase. The protons will spin the ATP synthase which produce ATP using Kinetic energy to escape. This occurs in cellular respiration as well, though the concentrated membrane is the inner membrane of the mitochondria rather than the plasma membrane.
The acidic or basic essence of a compound is often determined by the concentration of hydrogen ions which is calculated as pH, H+ and hydroxide anions are formed when water molecules break known as water self-ionization.
Q6) How is Hydrogen peroxide prepared?
A6) Hydrogen peroxide is the simplest kind of peroxide available (oxygen-oxygen single bond). It is a colourless liquid and is used in aqueous solution for safety reasons. It acts as a bleaching agent and is also used as a disinfectant. Concentrated hydrogen peroxide is a very reactive oxygen species and is used as a propellant in rocketry. The chemical formula for hydrogen peroxide is H2O2.
It is often referred to as water with one more oxygen atom. It is acidic in nature and PH is about 4.5. It is 100 percent degradable compound.
When barium peroxide is acidified and the excess water is removed by the process of evaporation under reduced pressure, we obtain hydrogen peroxide. The following reaction will clarify this:
BaO2.8H2O(s) + H2SO4(aq) → BaSO4(s) + H2O2(aq) + 8H2O(l)
Industrial Method of Preparation
Hydrogen peroxide is prepared by the electrolysis of 30% Ice -cold H2SO4. When acidified sulfate solution is electrolyzed at high current density, peroxodisulphate is obtained. Peroxodisulphate is then hydrolysed to get hydrogen peroxide.
2HSO–4(aq) [Electrolysis] → HO3SOOSO3H(aq) [Hydrolysis] → 2HSO–4(aq)+2H+(aq) +H2O2(aq)
Reaction Mechanism
2H2SO4 → 2H+ + 2HSO–4
At Cathode: 2H+ + 2e– → H2
At Anode:
2HSO–4 → H2S2O8 + 2e– ⇒ Peroxide Sulphuric Acid [Marshall’s acid]
H2S2O8 + H2O → H2SO5 + H2SO4 ⇒ Peroxomono Sulphuric Acid [Caro’s acid]
H2SO5 + H2O → H2SO4 + H2O2
Commercial Methods of Preparation
Auto-Oxidation Method:
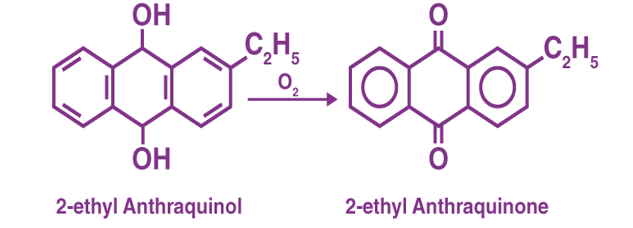
Q7) Define Gibb’s Free Energy
A7) Gibbs Free Energy is a measure of the potential for reversible or maximum work that may be done by a system at constant temperature and pressure. It is a thermodynamic function that was discovered in 1876 by Josiah Willard Gibbs to assume if a process will occur spontaneously at constant temperature and pressure. Gibbs free energy G is defined as
G = H - TS
where H, T, and S are the Enthalpy, temperature, and entropy. The SI unit for Gibb’s energy is the kilojoule.
Gibbs free energy combines both the enthalpy and entropy into a single value.
Gibbs free energy is the energy t with a that associates itself with a chemical reaction. It equals the enthalpy minus the temperature of the product and the entropy of the system.
G=H-TS
At constant temperature
ΔG = ΔH – TΔS
ΔG predicts the direction of a chemical reaction. If ΔG value is negative, then the corresponding reaction is spontaneous. If ΔG value is positive then reaction is non-spontaneous.
ΔGº=ΔHº-TΔSº
Where
ΔGº = Gibbs free energy (J or KJ)
ΔHº=enthalpy
T=Temperature
ΔSº=Entropy
Gibbs free energy is the energy that is available to do quality work.
Q8) What is the importance of Moh’s scale?
A8) There are several different methods to measure hardness Mohs’s hardness gives the ranking to Gemstones and other minerals. According to Mohs’s hardness, it is the ability of the material to resist abrasion or scratching.
The hardness of Gemstones and minerals are ranked by the common methods of Mohs’s (Mohs) scale. In 1812 a German mineralogist Friedrich Mohr devised this scale grades minerals on a scale from 1 (very soft) to 10 (very hard). As the Mohs scale is a relative one, it shows the difference between the hardness of a diamond and that of a ruby is much greater than the difference in hardness between calcite and gypsum. As an example, when diamond (10) is compared to Corundum (9) it is about 4-5 times harder, and 2 times harder than The Mohs rating may slightly differ depending on the individual sample the values will be ear same value. Half-numbers are used for in-between hardness ratings.
How to Use the Mohs Scale
A mineral with a given hardness rating will scratch other minerals of the same hardness and all samples with lower hardness ratings. As an example, if you can scratch a sample with a fingernail, you know its hardness is less than 2.5. If you can scratch a sample with a steel file, but not with a fingernail, you know its hardness is between 2.5 and 7.5.
Gems are examples of minerals Gold, silver, and platinum are all relatively soft, with Mohs ratings between 2.5-4. Since gems can scratch each other and their settings, each piece of gemstone jewellery should be wrapped separately in silk or paper. Also, be wary of commercial cleaners, as they may contain abrasives that could damage jewellery.
Mohs Scale of Hardness
Hardness | Example |
10 | Diamond |
9 | corundum (ruby, sapphire) |
8 | beryl (emerald, aquamarine) |
7.5 | Garnet |
6.5-7.5 | steel file |
7.0 | quartz (amethyst, citrine, agate) |
6 | feldspar (spectrolite) |
5.5-6.5 | most glass |
5 | Apatite |
4 | Fluorite |
3 | calcite, a penny |
2.5 | Fingernail |
2 | Gypsum |
1 | Talc |
Mohs Scale History
While the modern Mohs scale was described by Friedrich Mohs, the scratch test has been in use for at least two thousand years. Aristotle's successor, Theophrastus, described the test around 300 BC in his treatise On Stones. Pliny the Elder outlined a similar test in Naturalis Historia, circa AD 77.
Other Hardness Scales
The Mohs scale is only one of a number of scales used to assess mineral hardness. Others include the Vickers scale, Brinell scale, Rockwell scale, Meyer hardness test, and Knoop hardness test. While the Mohs test gauges hardness based on a scratch test, the Brinell and Vickers scales are based on how easily a material can be dented. The Brinell and Vickers scales are especially useful when comparing the hardness values of metals and their alloys.
Q9) Explain Roasting and Calcination?
A9) Calcination
Calcination is a very simple chemical process, where the solid material or the substance is heated in a controlled environment, in the process the temperature is also regulated. Calcination results a change in the substance both physically and chemically.
During calcination, the solid substances are subjected to heat at a very high temperature, this helps in removing volatile substances, water or oxidize the substance, hence the process is also called a purification process. The word Calcination has also been derived from the Latin word Calcinate which translates as “to burn lime”.
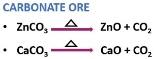
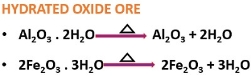
It is the chemical method of separating carbonate or hydrated oxide ores.
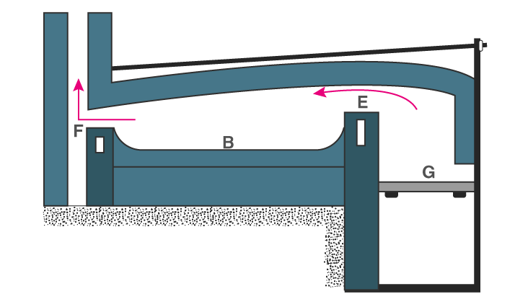
Calcination is usually carried out in furnaces, retorts, or kilns and often materials are racked over or stirred to make sure the product is uniform. One of the common arrangements that is used for calcination is the reverberatory furnace.
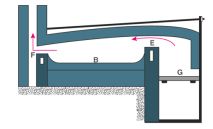
Calcination Process
The construction of the reverberatory furnace is different, however the in most cases the flames and hot gases from the fire come in direct contact with the material that is to be calcinated, but the fuel is separated from them.
In the above figure, the fire burns on the grate at G. Now the flames passing over the bridge at E are deflected downward by the low sloping roof of the furnace and pass directly over the surface of the charge or the material under calcination which is laid on the platform B. The fumes and hot gases then escape through the throat F into the chimney. The charge is spread out evenly on the bed as a thin layer.
A carbonate ore produces carbon dioxide under heat exposure.
The hydrated oxide ore releases water under heat exposure.
Roasting
In roasting, the ore concentration or the ore is subjected to very hot air, this method is generally applied to sulfide minerals. The process proceeds under the influence of heat and air. During roasting, the sulpide ore is heated at a temperature below the sulpide melting point.
For example: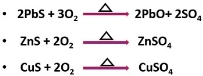
Leaching
It is a chemical method, in which the ore is treated with the suitable reagent to solubilize the ore.

The soluble ore or mineral can be separated from the insoluble gangue. After the separation of ore, it can further be recovered by several chemical methods.
Q10) Explain Zone refining?
A10) Scientist W.G Pfann discovered this refining process against impurities , typically metals that are purified using this method are impurities and other metals.
The impurities are higher solubility in the molten metal as compared to that in solid metal, the difference in solubility of impurities makes this process possible.
Zone refining is a technique to get highly pure crystals of impure elements (specially metals) by using melting and crystallization processes. In this narrow region of the crystal is heated so that impurities get melted and forms a molten zone which moves along the crystal.
Principle of zone refining
Zone refining technique is based on the fact that impurity has more solubility in molten state, because of this when a molten metal crystallizes on cooling, impurities are automatically excluded as they do not form part of the pure crystal.
In zone refining, impurities are removed by moving the molten zone through heater and recrystallized pure metal is left behind in solid form, in this zone should be moving slowly as possible to get highly pure metal.
Zone Refining process
In the zone refining process,a mobile heater is fixed at one end of te impure metal rod which is to be purified, now an impure metal rod fitted with a circular mobile heater is fixed in a column filled wth inert gas.Tis circular heater heats the rod radially and produces a region in which the temperature is uniformly elevated to the melting point I a plane perpendicular to the axis of the rod. As the heater moves along the rod, the molten zone also propagates down the rod. We can make the heater move very slowly, because of this impurities and pure metal both get melted but atoms of pure metal recrystallize while molten impurities move with heater or molten zone, thus after the process impurities gets collected at one end while solid pure metal remains at another end, the process is repeated many times to get pure metal.
Applications of Zone refining
Limitations of Zone refining
Zone refining is an expensive process, because of tis applications are limited to lab reagents and valuable chemicals. Sometimes when solid-liquid equilibria are not favourable for all impurities then we have to combine zone refining with the techniques to achieve ultra-high purity.
Scientist W.G Pfann discovered this refining process against impurities, typically metals that are purified using this method are gallium, silicon, indium, germanium, boron and other metals.
The impurities are higher solubility in the molten metal as compared to that in solid metal, the difference in solubility of impurities makes this process possible.
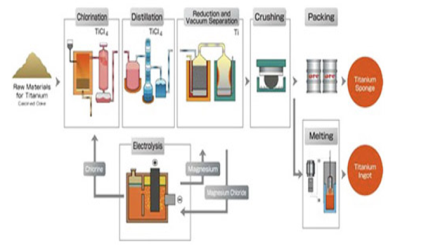
Q11) Explain the Kroll’s process?
A11) Titanium is a remarkable metal. The metal is strong and durable yet flexible and light, the metal is biocompatible and corrosion resistant. The exceptional properties of titanium have its wide applications in medicine to defence, airplanes and dental implants.
Titanium was first discovered in the 1700s, but was not identified and given a name until the early 1900’s.
The word Titan and its metal alloy has led to the name of titanium and has lived up to its name, it has been the driving force for much of the world’s technology and equipment.
The metal alloy is found in the earth in plenty and the last few decades it is easily extracted from earth. Crude titanium ore cannot be used in its natural form. It must be taken through a tedious and time-consuming process that uses dangerous chemicals and costly equipment.
The process of transforming crude titanium from the earth (known as ore) to the type of metal we use in many aspects of our lives is called the Kroll Process.
The Kroll Process is named after William J. Kroll, who invented the process in 1940 to produce Zirconium
The Kroll Process is comprised of 6 steps to produce the titanium we have come to use and benefit from:
Step 1: The pure titanium is changed into sponge by passing an electric charge through the ore, this process occurs in a chlorinator, the chlorine gas is then passed through a charge.
Step 2: Titanium tetrachloride is formed by removing an oxygen resulting in the formation of titanium tetrachloride. This crude form of titanium tetrachloride is then purified through fractional distillation.
Step 3: Once distillation is complete, magnesium or sodium is added to the pure titanium tetrachloride to form a metallic sponge and either magnesium chloride or sodium chloride is liberated.
Step 4: The newly formed metallic sponge is then crushed and pressed.
Step 5: The crushed titanium sponge is then melted in an electrode vacuum arc furnace at extremely high temperatures.
Step 6: Each batch presenting the furnace is known as ingot and weighs up to 12000 pounds, the melted titanium is allowed to harden and solidify in the furnace rather than being poured out.
Titanium was once flaunted as the metal that belongs to the future, it has played a major role in various advancements in the field of aerospace, defence, manufacturing, medical, transportation and leisure industries.
The comfort and luxury we enjoy today would not be possible without the discovery of Kroll’s process.
Q12) Which process is called the crystal bar process and supercedes the Kroll’s process?
A12) The process was first discovered by a Dutch chemist Anton Eduard van Arkell in 1952, the process is used to produce pure metal like tungsten, titanium and zirconium by forming metal iodides followed by thermal decomposition on a hot tungsten.
It is used in the purification of titanium and zirconium, impure Ti/Zr is heated in ana evacuated vessel with iodine, the volatile titaniumtetreiodide/zirconium teteraiodide is the final product which is finally heated with a tungsten filament to acquire pure metal.
The process is also known as iodide process or the crystal bar process, this process was the first industrial production of ductile metallic zirconium in its pure form, this is used in small quantites for the production of ultra pure zirconium and titanium. The process succeeded the kroll process.
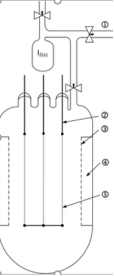
Fig 21:An apparatus used for the crystal bar process. The main body is made of quartz glass. (1) to vacuum pump, (2) 6 mm molybdenum electrode, (3) molybdenum net, (4) chamber for the raw metal, (5) tungsten wire
The impure form of thorium, protactinium, vanadium, hafnium, titanium and zirconium are heated in an evacuated vessel along with a halogen at a temperature of 50-250oC. the process clearly depicts the involvement of Til4 and Zrl4, which become volatile leaving behind the impurities s solid. At atmospheric pressure TiI4 melts at 150 °C and boils at 377 °C, while ZrI4 melts at 499 °C and boils at 600 °C. At reduced pressure the boiling points are low. The gaseous metal tetraiodide is decomposed on a white-hot tungsten filament (1400 °C). As more metal is deposited the filament conducts better and thus a greater electric current is required to maintain the temperature of the filament. The process can be performed in the span of several hours or several weeks, depending on the particular setup.
Can take place using a number of metals and also any halogen or a combination of halogens that is appropriate for the transport mechanism and the reactions involved. The titanium, zirconium and hafnium are the only metals used on an industrial scale due to its pure form. They are used extensively in small scale industries for specific purity needs.
Q13) Which process is used to purify Nickel?
A13) The Mond process is a method for purifying nickel. In this lesson we will learn what this process is, how it works, and what applications it is used for.
One of the common types of rechargeable batteries uses nickel as the main ingredient to allow it to work. But nickel that is simply mined doesn't work, because it isn't pure nickel. Thus, it needs to be refined. One process for refining nickel is the Mond process (also called the carbonyl process)
The Mond process was developed by Ludwig Mond, a German-British chemist, in the late 19th century. He discovered that metals can form carbonyls under specific conditions, and thus discovered methods for refining metals
The Reaction
There are three reaction steps in the Mond process. Nickel typically comes in the form of nickel oxide, with other impurities. In step 1, syngas, which is a mixture of hydrogen gas and carbon monoxide gas, is added to the nickel. The nickel oxide and impurities react with the hydrogen gas to form an impure solid nickel:

Although the reaction makes it appear as though we have pure nickel, it is not pure at this point
Next, the carbon monoxide will react with the impure nickel. But only nickel will readily react with the carbon monoxide - the other metals won't. The nickel and carbon monoxide reacts to form nickel carbonyl gas, but the impurities remain as solids:

Finally, the nickel carbonyl gas is heated, causing the nickel carbonyl to decompose (break apart) into pure nickel and carbon monoxide:
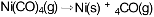
Them the carbon monoxide is recycled to continue the process and the nickel is collected
Q14) Write a note on Lithium?
A14) Lithium occurs in the alkalis of the periodic table it is a chemical element.
Uses of Lithium
Properties of Lithium
Lithium is found only in salts and minerals. The lithium-ion battery is a key component that is used in many digital devices.
Q15) Write the uses of lead (Pb)?
A15) Lead is a corrosion resistance metal, used in the manufacturing of pipes paints, hair dyes etc. They are also added to petrol as an anti-knocking agent to improve the performance of engine. However, these uses have been limited due to the toxic effects of Pb.
Batteries with lead acid are utilised to operate automobiles, submarines and emergency generators have a long life and low impedance.
The element is applicable in the ammunition industry for making shots and bullets.
Lead-sheathed cables and wires are used in petrochemicals plants as very good chemical barriers against hydrocarbons and moisture.
It is used in pencils, flashings, lifting weights, anchors, and diving weight belts
Glasses coated with its oxide are used in making windows (known as lead lights) and roofing. Pb crystal glasses are sometimes utilized as storage containers for corrosive liquids.
Solder joints comprising of lead and tin in equal amounts are used in joining pipes and electrical components. There are other alloys of the metal as well used in printing plates, presses, and bearings.
Q16) Write the importance of Halides?
A16) Halides are binary compounds that are of which one part is an element another part is the halogen atom. A radical is less electronegative compared to that of halogen which form astatine, bromide, fluoride and chloride. Most of the salts are halides. Various halide compounds are tested using silver nitrate solution. Some include Kl, KBr, and KCl.
When halogen reacts with silver nitrate solution, precipitation will be formed, and it varies in colour depending upon the type of halides. Some include Silver Fluoride with no precipitate. Silver Bromide with pale yellow precipitate. Silver Iodide with green precipitate. Silver Chloride with a white precipitate.
Halogen Atom comprising a negative charge is termed as the halide ion. Halide mineral includes halide anion. Fluorite and Halite are two important halide minerals. Fluorite is the main source of hydrogen fluoride. Halite is a primary source of sodium chloride. Bischofite forms a primary source of magnesium. Many of the halides are present in the marine evaporite deposits. Few of the halide anions include iodide, bromide, chloride, and fluoride.
Example of Halide Compounds
Organic Halide compounds consist of one or more halogens and belong to a class of synthetic and natural chemicals. Some examples of halide compound include calcium chloride, silver chloride, potassium iodide, potassium chloride, sodium chloride, Iodoform, Chlorine Fluoride, Organohalides, Bromoethane and more.
Metal Halides
Metal Halides are compounds between a halogen and metals. Some are covalently bond, and some are ionic. Covalently bonded metal ions may form polymeric structures. Metal Halides are formed when all halogens react with metal. It is stated in the below equation.
2M+nX2→2MXn
Uses of Halides
Q17) Highlight the properties of Tin(Sn)?
A17) Properties of Tin
Allotropes of Tin (Sn element)
Health Effects of Tin
Q18) Explain Electrolytic reduction?
A18) Electrolytic Reduction. The highly electropositive elements such as alkali metals, alkaline earth metals and aluminium cannot be extracted by carbon reduction methods. They are extracted by the electrolysis of their fused salts. The process of extraction of metals by the use of electrolysis phenomenon is called electrometallurgy.
For example, sodium metal is extracted by electrolysis of molten sodium chloride containing other salts as impurities.
NaCI(l) → Na+ + Cl–
At cathode Na+ + e– → Na
At anode 2Cl‑ → Cl2 + 2e–
Q19) Define Hydrometallurgy?
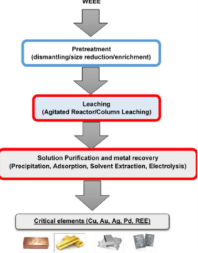
A19) This is an extraction method involving metal or metal compounds from their ores, by pre-treatments that include the use of a leaching agent, separation of impurities and precipitation. It is used in the extraction of uranium, gold, zinc, silver and copper from low-grade ores. Hydrometallurgy has been improved by the development of process like solvent extraction and ion exchange.
Hydrometallurgy involves the recovery of the metals from a range of metal bearing sources. They involve a combination of extractive metals, sciences technology and chemistry for the recovery of metals from a wide variety of metal-bearing sources, that include ores, solutions, recycled materials, waste streams, intermediates and mineral concentrates that are converted into useful products for the society.
This wider field of technology is efficient in the on-site production of metals and forms an integral part of a growing number of metallurgical processes. It involves research, studies and novel technologies to obtain pure metals and better options for metal extraction. Hydrometallurgy revolves around three processes: leaching, metal recovery and solution purification.
The various techniques used in these processes include:
Q20) Write a note on Ion exchange Solvent extraction?
A20) Ion exchange Solvent Extraction
Solvent extraction, also known as liquid-liquid extraction, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent.
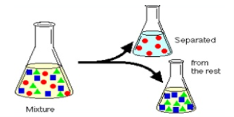
Fig 13: Extraction of solvent
Importance of the process
Solvent extraction consists of transferring one or more solutes contained in a feed solution to another immiscible liquid (solvent), the solvent that is enriched in solute is called Extract and the feed solution that is depleted of solute is called Raffinate
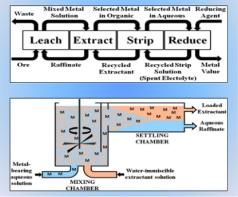
Fig 14: Solvent extraction as a part of Hydrometallurgy
Types of Solvent Extraction
Two Basic steps of Solvent extraction
1. Extraction
UO2(SO4)4- + 2(R3NH)2SO4 = (R3NH)4UO2(SO4)3 +2(SO42-)
2. Stripping
(R3NH)4 UO2 (SO4)3 + Na2CO3 = R3N +Na4UO2(CO3)34- + H2O + Na2SO4
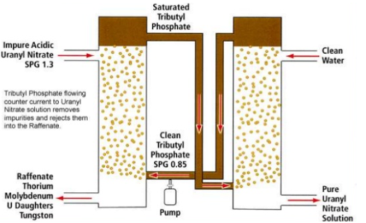
Fig 15: Solvent Extraction of Uranium
Single stage Solvent extraction
This process is commonly followed in the labs, contacting is carried out by taking two phases together in a separating funnel followed by vigorous agitation so that the phases may disperse in each other as fine droplets. Ceasing the agitation lead to the separation of phases in two distinct layers.
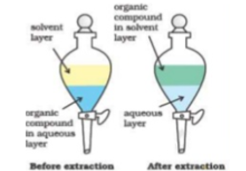
Fig 16: Single stage extraction
Multi Stage Counter Current Solvent Extraction
Applications
Today the process of solvent extraction is widely applied in miscellneous fields of sceince and techology
The hydrometallurgical processing of complex ores/ concentrates/ secondaries/ wastes, the solutions containing different non-ferrous metals are obtained during leaching the materials in acidic, ammoniacal or alkaline lixiviants. The spent solutions and effluents are also generated in different process industries and contain metallic values. Their discharge in the sewage or river is major concern for environment. Solvent extraction (SX) is one such proven technique in the hydrometallurgical processing for selective extraction and separation of metals due to the ease of applicability, versatility and ability to produce high value products. It is used on commercial scale for the recovery of different metals from different solutions viz. copper, nickel, cobalt, zinc, tungsten molybdenum, uranium, rare earths etc. The effluents from waste streams are also processed to recover metals using organic extractants. In the solvent extraction process, the extraction of metal ions, or uncharged species in the aqueous phase takes place by ion pair transfer, ion exchange with the extractant. In the case of ion-pair transfer, electrically neutral molecules interact with the extractant to form an addition compound. The most suitable extractants for such interaction are those having an oxygen atom with a lone pair of electrons viz. ether, alcohols, and the neutral phosphoric acid esters. For example, tri-butyl phosphate extracts uranium from nitric acid solutions as follows:

Similarly, the metal is transferred from the aqueous phase as simple ion, and at the same time an ion from the extractant is transferred stoichiometrically to the aqueous phase in the ion exchange process. The ion present in the aqueous phase may be either cationic or anionic form. The extraction of metal takes place by cation or anion exchange mechanism.
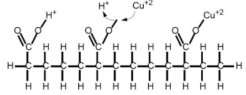
Fig : Carboxyl groups exchanges the ion it currently holds (H+) for a Cu2+ ion, the Cu2+ is later released by contacting it with a stripping solution (very high H+ conc.)