Question Bank
Unit 06
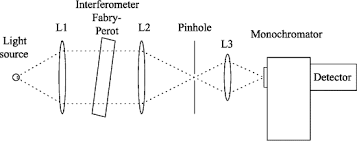
Q-1Explain about Monochromator with the help of diagram.
Answer: Monochromator used to measure the wavelength of the light. Monochromator is placed between the sample and the source of light passing from that the light passes to the detector. Here the monochromatic used to detect the wavelength of the light form the source. While on moving further there is a Double beam Ultraviolet-Visible instrument is used here is a splitter and a series of mirrors to get the beam to a reference sample and the sample to be analyzed, this allows for more accurate readings. In the simultaneous Ultraviolet-Visible instruments monochromator is removed hence it has diode array detectors that allow the instrument to simultaneously detect the absorbance at all wavelengths. This instrument is much faster and accurate than double and single Ultraviolet-Visible instrument.
Q-2Mention the application of electronic spectroscopy.
Answer: Applications:
1- This is majorly used in the analytical chemistry.
2- This can be used as a detector for the High performance liquid chromatography.
3- Ultraviolet-Visible Spectroscopy is used as the semiconductor in the industry to measure the thickness and optical properties of wafer.
Q-3What are different type of electronic spectroscopy?
Answer: The absorption of UV or visible radiation corresponds to the excitation of outer electrons. There are three types of electronic transition which can be considered;
- Transitions involving p, s, and n electrons
- Transitions involving charge-transfer electrons
Q-4Explain rotational spectroscopy.
Answer: Rotational Spectroscopy is also called as the microwave spectroscopy. It is the measurement of energy of transition between quantized rotational states of molecules in the gas phase. The rotational spectra of non-polar molecules cannot be observed by this method while it can be measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. The molecules of the Rotational spectroscopy are classified according to their symmetry. The important feature of molecular rotational spectroscopy for chemical analysis is that the resolution of the spectral transitions is sufficiently high that the spectra of isomers with only small changes in mass distribution can be measured without spectral overlap. For example, the 13C- isotopologues of molecules, isomers created when a single carbon atom is isotopically substituted, are routinely resolved in the instruments used for rotational spectroscopy. The extreme sensitivity changes to in the mass distribution make rotational spectroscopy well-suited to distinguishing diaestromers of molecules with multiple chiral centers. A second advantage of the high-resolution of the spectrometers is that complex sample mixtures can be analyzed directly without spectral overlap. An example of the rotational spectrum from the vapor head space of an essential oil will be presented later to illustrate this capability.
Q-5Mention the applications of rotational spectroscopy.
Answer:
1- Rotational Spectroscopy is used in the determination of molecular structure in the gas phase molecule.
2- Rotational Spectroscopy also provide the information about the electronic structures of molecule.
3- This plays the major role in exploration of chemical composition of interstellar medium.
Q-6Explain the applications of vibrational spectroscopy.
Answer: 1-Infrared technique is used in organic and inorganic chemistry.
2-This is used in quality control, dynamic measurement.
3-This is majorly used in the forensic labs.
4-Infrared Spectroscopy is used in measuring the degree of polymerization in manufacturing the polymers.