UNIT 1
Question Bank
Q1- What is the difference between hard and soft water?
A- Hard water is the excessive presence of calcium and magnesium. The higher amount of these minerals that are present in water, the higher it will rank on the hard water scale. While both essential to everyday health, calcium and magnesium are not essential for water usage.
Soft water is free of harsh minerals. These water are calcium and magnesium free, soft water can prevent scale buildup around home. Best of all, it increases the effectiveness in soap compared to hard water which can inactivate the soap’s ingredients. In regard to health, soft water can combat the dryness and stickiness caused by cold weather, low humidity, and hard water.
Q2-What are different types of hardness?
A-
Temporary hardness (carbonate) :-
- When water containing calcium and magnesium bicarbonates is heated, bicarbonate decompose and from insoluble carbonate and form hydroxide.
- On filtering such water, soft water is obtained.
- The hardness which can be removed by more boiling is referred as ‘temporary hardness ’ or bicarbonate hardness.

 Ca
Ca
Mg 
 Mg
Mg + 2 CO
+ 2 CO 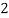
Permanent hardness :-
- The term permanent hardness or non-carbonate is the term applied to the hardness caused by dissolved chlorides , nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate , calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
4. As the sulphate and chloride are neutral salts , the hardness caused by presence of calcium and magnesium sulphate , chlorides and nitrates is termed as non alkaline hardness or non carbonate hardness.
Q3- What do you understand by coagulation?
A- Coagulation treatment chemicals are used in effluent waste water treatment process for solids removal, water clarification, lime softening, sludge thickening and solid dewatering. It is the process which can be used as preliminary steps between waste water treatment process like filtration and sedimentation. This can be affected by its dose, its pH and mass. Coagulation is usually accomplished in two stages i.e, rapid mixing and slow mixing.
Q4- Write down the properties of Ozone?
A-
(i) They are pale blue gas slightly soluble in water.
(ii) It formed dark blue liquid when condenses.
(iii) It forms violet-black solid at temperature below 80K.
Q5- Mention the difference between sludge and scale.
sludge | scale |
Sludge is loose deposite of slimy matter | Scale is hard coating |
Sludge is less adherent on boiler metal and can br removed by brushes detergents | Scale is strongly adhered to boiler metal and difficult to remove |
Heat transfer to boiler water is affected slightly | Being bad conductor heat transfer to boiler water is affected largely |
Sludge form at coller parts and flow rate is low | Scale is formed at hotter parts |
Sludge may lead to choking | Scale may lead to bluging of metal tube its bursting explosion |
Q6- What are zeolites? Discuss the zeolite process of softening of hard water.
A-
Processes :-
For softening of water by zeolite processes hard water is percolated at a specified rate through a bed of zeolite kept in a cylinder (shown in fig.)
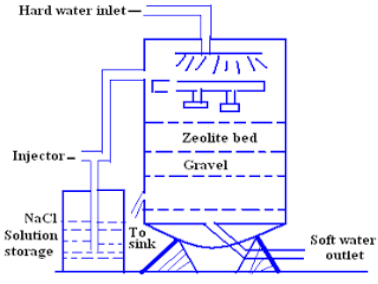
The hardness causing ion are retained by the zeolite as caze and MgZe while the out-going water contain sodium salts reaction take place during the softening processes are
Ca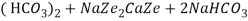
Mg
Ca
M
Regeneration-
After some time, the zeolite is completely converted into calcium and magnesium zeolite and it ceases to soften water,i.e. it gets exhausted.
At this stage the supply of hard water is stopped and the exhausted zeolite is reclaimed by treating the bed with a concentrated ( 10 percent ) brine ( NaCl ) solution.
The washings ( containing CaCl2 and MgCl2 ) are led to drain and the regenerated zeolite bed thus obtained is used again for softening purpose.
Limitations of zeolite processes :-
- If the supply of water is turbid the suspended matter must be removed (by coagulation, factorization, etc.) before the water is admitted to the zeolite bed , otherwise the turbidity will clog the processes of zeolite bed there by anking it inactive.
- If water contains large quantities of colored ions such as Mn2+ they must be removed first because these ions produce manganese and iron zeolite which cannot be easily regenerated.
- Mineral acids if present in water destroy the zeolite bed and therefore they must be neutralized with soda before admitting the water to the zeolite softening plant.
Advantages of zeolite processes:-
1. It removes the hardness almost completely and water of about 10 ppm hardness is produced
2. The equipment used is compact occupying a small space
3. No impurities are precipitated so there is no danger of sludge formation in the treated water at later stage
4. The processes automatically adjust itself for variation in hardness of incoming water
5. It is quite clean
6. It requires less time for softening
7. It requires less skill for maintenance as well as operation
Q7- Calculate the amount of lime and soda required for softening of 15000lt. Of water which analyzed as follows: temporary hardness = 25 ppm, permanent hardness = 20ppm permanent Mg hardness = 15 ppm.
Solution:
Lime Requirement
= 74/100 (Temp hardness + Perm. HMg Hardness) * vol. Of water
= 74/100(25 + 15) * 15000
= 444gm.
Soda requirement
=106/100 (permanent hardness)*volume of water
=106/100*20*15000
=318gm
Q8-A zeolite softener was 90% exhausted by removing the hardness completely when 100000 lt. Of hard water sample passed through it. The exhausted zeolite bed required 150 lt of 30% NaCl solution for its complete regeneration. Calculate the hardness of water.
A-
150 lt of 30% NaCl solution required= 150*30
= 4500 gm NaCl
W=4500 gm
E=58.5
Equivalent of CaCO3 = W*50/E
= 4500*50/58.5
=3846 gm CaCO3
100000 lit. Water = 3846 gm CaCO3
= 3846*1000 mg CaCO3
90% exhausted by removing the hardness
100000 lt. Water = 3846*1000*100/90 gm CaCO3
1lt. Water= 3846*1000*100/90*100000
= 3846/90
=42.73 ppm
Q9-1000 litres of hard H2O are softened by zeolite process. The zeolite was regenerated by passing 20 litres of sodium chloride solution containing 1500 mg/lit. Of NaCl. Calculate hardness of H2O.
Solution:
20 litres of NaCl contain = 1.5 * 20 = 30 gm of NaCl
We have to convert it in terms of CaCO3 equivalent.
2 NaCl = CaCO3
2* 58.5 gm = 100 gm
58.5 = 50 gm
Thus, 30 gm of NaCl equivalent to 30*50/58.5 gm of CaCO3
10,00 litres of H2O contains 30*50/58.5 gm of NaCl as CaCO3
1 litre of H2O contains 30/1000*50/58.5 gm of NaCl as CaCO3
Thus, 1 litre water contains = 30/1000*50/58.5*1000 mg of NaCl as CaCO3
= 25.64 mg 1 litres
Q10-A completely exhausted zeolite requires 120 lt. Of sodium chloride solution having 100 gm/lt. Of NaCl. How many litres of a water having hardness 500 ppm be softened by the zeolite?
Solution-
1 lt. Of NaCl solution = 100 * 120
=12000 gm of NaCl
12000*50/58.5 gm of CaCO3 eq. Hardness
Hardness of water= 500 ppm or 500 mg/lt
=0.5g/lt
Thus, 0.5 of hardness = 1 lt of hard water
=12000*50/58.5 gm of hardness
=1*12000*50/58.5*0.5 lt.
=20518 lt. Of water