Unit 3
Question bank
Q.1Describe three-dimensional crystal system and their Bravais lattices?
Answer:
If the atoms or molecules are uniquely arranged in crystalline solid or liquid, we call it as a crystal structure. A crystal possesses long range order and symmetry. The main property of crystal structure is its periodicity. This periodicity is due to the arrangement of atoms/molecules in the lattice points
Some common crystal structures you should know
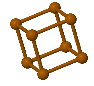 Simple Cubic | 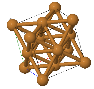 Face Cantered Cubic | 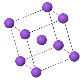 Body Centered Cubic | 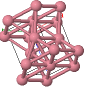 Hexagonal Close Packed | 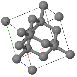 Diamond |
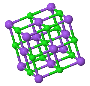 NaCl | 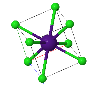 CsCl | 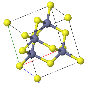 Zincblende | 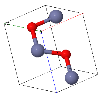 Wurtzite | 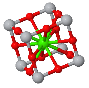 Perovskite |
Bravais Lattice
In solid state physics one usually encounters lattices which exhibit a discrete translational symmetry. If one considers for instance the vector space R3 this means that a translation of the whole lattice by any translation vector given by.
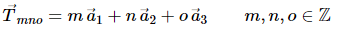
A lattice that can be characterised in this way is referred to as a Bravais lattice. All lattice points are equivalent, However, if there are lattice points with different environments they cannot form a Bravais lattice.
Q.2 What are miller indices?
Answer:
It is necessary to have some method of identifying and visualising the most useful planes. Crystallographers use an identification system referred to as Miller, or hkl, indices (a sort of zip-code for the planes).
Miller indices are simply a set of three numbers, hkl, which can take on any combination of three integer values between +∞ and −∞, e.g. (111), (-501) and (7-2-2). Each combination of hkl describes a unique set of planes filling the crystal and so hkl is often presented as a subscript to a property: e.g. dhkl which therefore means the d spacing between the planes defined by hkl. The Miller indices, hkl, also provide a useful means for visualising the planes. The convention is that if you have three axis, x, y, z, with three unit spacings, a, b, c, on each then one of these hkl planes can be visualised as the plane which intersects the x, y, z-axes at distances of a/h, b/k, c/l respectively
Q.3 What is a Space lattice, unit cell, Basis of a crystal? What is the coordination number and packing fraction?
Answer:
Basis:
A basis is a collection of atoms in particular fixed arrangement in space. We could have a basis of a single atom as well as a basis of a complicated but fixed arrangement of hundreds of atoms
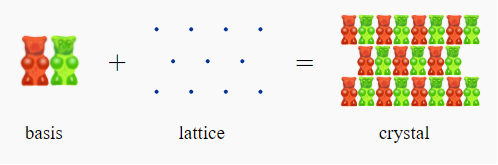
Unit cell:
In general, the unit cell is chosen such that it is the smallest unit cell that reflects the symmetry of the structure. There are two distinct types of unit cell: primitive and non-primitive.
The coordination number (CN) is the number of nearest neighbours of a given particle in the crystal lattice. It determines the nature of the bonding in a crystal. The most common coordination numbers are 4, 6, 8, and 12.
Packing efficiency is the fraction of the unit cell that is occupied by particles. Closest packed spheres pack with a packing efficiency of 74%.
Spheres cannot be packed without creating some void space, but the amount of void space depends upon how well they are packed. Packing efficiency (PE) is that fraction of the unit cell volume that is actually occupied by particles, not void space., the packing efficiency of a unit cell is
Packing Efficiency | = |
× 100% | ||
|
|
| ||
|
|
| ||
PE | = |
× 100% |
a = the length of a side of the unit cell, so a3 is the volume of the unit cell.
Q.4 Explain NaCl crystal structure with a neat labelled diagram showing space lattice and the basis?
Answer:
Sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contain 39.34 g Na and 60.66 g Cl.
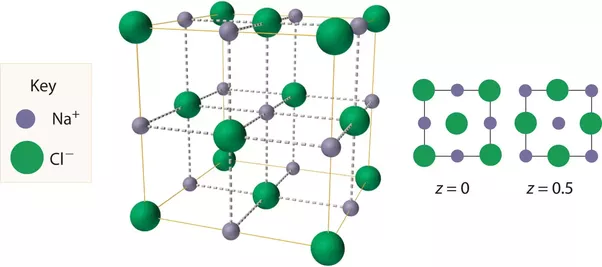
The salient features of its structure are:
- Chloride ions are ccp type of arrangement, i.e., it contains chloride ions at the corners and at the centre of each face of the cube.
- Sodium ions are so located that there are six chloride ions around it. This equivalent to saying that sodium ions occupy all the octahedral sites.
- As there is only one octahedral site for every chloride ion, the stoichiometry is 1: 1.
- It is obvious from the diagram that each chloride ion is surrounded by six sodium ions which are disposed towards the corners of a regular octahedron. We may say that cations and anions are present in equivalent positions and the structure has 6: 6 coordination.
- The structure of sodium chloride consists of eight ions a unit cell, four are Na+ ions and the other four are Cl– ions.
Q.5 Obtain the relation between the cell edge and the atomic radius in the case of a boc unit cell.
Answer;
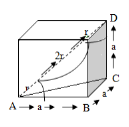
From the geometry, we can write
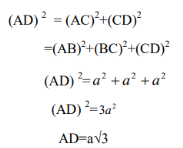
The diagonal of the cube AD=4R
Therefore
4r= a√3
r=a. √3/4
Q.6 Define following:
- Atomic radius
- Co-ordination number.
- Unit cell.
- Lattice planes
Answer:
Lattice planes:
A set of parallel and equally spaced planes in a space lattice, which are formed with respect to the lattice points are called lattice planes.
Unit cell:
The unit cell is defined as the smallest geometric figure, the translational repetition of which in all over the three dimensions gives the actual crystal structure
(OR)
The unit cell may also be defined as the fundamental elementary pattern with minimum number of atoms, molecules (or) groups of molecules which represents the total characteristics of the crystal.
Atomic radius:
Atomic radius is defined as half of the distance between any two nearest neighbour atoms which have direct contact with each other, in a crystal of a pure element. It is interns of cube edge ‘a’. 24
Co-ordination number:
Co-ordination number is the number of nearest neighbouring atoms to a particular atom. (Or) Co-ordination number is the number of nearest neighbours directly surrounding a given atom
Q.7 Define Atomic packing factor (or) Packing density (or) density of packing
Answer:
Atomic packing factor is defined as the ratio between the volume occupied by the total number of atoms per unit cell (v) to the total volume of the unit cell (V).

APF=v/V
Q.8 A metal is found to have BCC structure, a lattice constant of 3.31 Å, and a density of 16.6 g/cm3. Determine the atomic weight of this element.
Answer
BCC structure, so n = 2
a = 3.31 Å = 3.31 x 10–10 m
= 16.6 g/cm 3
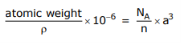
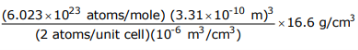
So atomic weight=181.3g/mol
Q.9 Determine the total void volume (cm3 /mole) for gold (Au) at 27oC; make the hard-sphere approximation in your calculation, and use data provided in the periodic table.
Answer:
First determine the packing density for Au, which is FCC; then relate it to the molar volume given in the periodic table
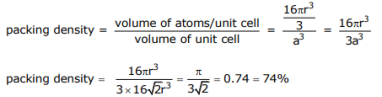
Void volume = 1 – packing density = 26%
From the packing density (74%) we recognize the void volume to be 26%. Given the molar volume as 10.3 cm3 /mole, the void volume is:
0.26x 10.3 cm3 / mole = 2.68 cm3 / mole
Q.10 Define Bragg’s law for X - ray diffraction and explain?
Answer:
Bragg’s Law can be derived using simple geometry by considering the distances travelled by two parallel X-rays reflecting from adjacent planes. The X-ray hitting the lower plane must travel the extra distance AB and BC. To remain in phase with the first X-ray, this distance must be a multiple of the wavelength thus:
nλ = AB+BC = 2AB (since the two triangles are identical)
The distance AB can be expressed in terms of the interplanar spacing (d) and incident angle (θ) because d is the hypotenuse of right triangle ZAB shown at right.
Sin(θ) = AB/d
Thus AB = d sin(θ)
Therefore:
nλ = 2 d sin(θ)
Reflection (signal) only occurs when conditions for constructive interference between the beams are met
These conditions are met when the difference in path length equals an integral number of wavelengths, n. The final equation is the
BRAGG’S LAW n λ = 2 d sin θ
