Unit - 1
Introduction to materials
Q1) What is material?
A1)
- Material choice is a totally essential step withinside the method of gadget layout. Selection of cloth relies upon following aspects: Performance Requirements: Material is chosen thinking about the layout constraints and overall performance necessities e.g. Hundreds performing at the member, size & weight constraints, environmental conditions, preferred reliability & sturdiness etc.
- Material Properties: Performance necessities are as compared with homes of substances to choose the nice appropriate cloth. For instance pressure degree expected for any gadget member is as compared with the power of the to be had substances.
- Properties may be Physical (melting point, co-green of thermal expansion, thermal conductivity, particular heat, particular gravity, electric conductivity, magnetic homes etc.), Chemical (corrosion resistance, reactivity with acids, bases, water etc.), Mechanical (hardness, toughness, ductility, malleability etc.) or Manufacturing (castability, weldability, formability, machineability etc.).
- Any of those homes can grow to be essential relying upon the layout necessities and environmental conditions.
- Manufacturing Aspects: Along with the choice of cloth fashion dressmaker additionally has to determine approximately the producing approaches for use to provide it preferred form.
- Therefore further to the producing homes of the cloth, production constraints also are to be taken care of, even as choosing a selected cloth.
- Availability & Cost: Material decided on need to be without difficulty to be had at a suitable fee. In addition to the cloth fee, general fee of fabrication is likewise taken into consideration because the preferred form has to receive to the cloth with nice pleasant and least fee. Availability of a massive quantity of substances with various homes makes the activity of cloth choice very difficult.
Q2) Classify the metal in detail.
A2)
Engineering materials refers to the group of materials that are used in the construction of manmade structures and components. The primary function of an engineering material is to withstand applied loading without breaking and without exhibiting excessive deflection. The major classifications of engineering materials include metals, polymers, ceramics, and composites. The important characteristics of the materials within each of these classes are discussed on this page, and tables of material properties are also provided.
Metals
Metals are the most commonly used class of engineering material. Metal alloys are especially common, and they are formed by combining a metal with one or more other metallic and/or non-metallic materials. The combination usually occurs through a process of melting, mixing, and cooling. The goal of alloying is to improve the properties of the base material in some desirable way.
Metal alloy compositions are described in terms of the percentages of the various elements in the alloy, where the percentages are measured by weight.
Ferrous Alloys
Ferrous alloys have iron as the base element. These alloys and include steels and cast irons. Ferrous alloys are the most common metal alloys in use due to the abundance of iron, ease of production, and high versatility of the material. The biggest disadvantage of many ferrous alloys is low corrosion resistance.
Carbon is an important alloying element in all ferrous alloys. In general, higher levels of carbon increase strength and hardness, and decrease ductility and weldability.
Carbon Steel
Carbon steels are basically just mixtures of iron and carbon. They may contain small amounts of other elements, but carbon is the primary alloying ingredient. The effect of adding carbon is an increase in strength and hardness.
Most carbon steels are plain carbon steels, of which there are several types.
Low-Alloy Steel
Low-alloy steels, also commonly called alloy steels, contain less than about 8% total alloying ingredients. Low-alloy steels are typically stronger than carbon steels and have better corrosion resistance.
Some low-alloy steels are designated as high-strength low-alloy (HSLA) steels. What sets HSLA steels apart from other low-alloy steels is that they are designed to achieve specific mechanical properties rather than to meet a specific chemical composition
Tool Steel
Tool steels are primarily used to make tooling for use in manufacturing, for example cutting tools, drill bits, punches, dies, and chisels. Alloying elements are typically chosen to optimize hardness, wear resistance, and toughness.
Stainless Steel
Stainless steels have good corrosion resistance, mostly due to the addition of chromium as an alloying ingredient. Stainless steels have a chromium composition of at least 11%. Passivation occurs with chromium content at or above 12%, in which case a protective inert film of chromic oxide forms over the material and prevents oxidation. The corrosion resistance of stainless steel is a result of this passivation.
Cast Iron
Cast iron is a ferrous alloy containing high levels of carbon, generally greater than 2%. The carbon present in the cast iron can take the form of graphite or carbide. Cast irons have a low melting temperature which makes them well suited to casting.
Gray Cast Iron
Gray cast iron is the most common type. The carbon is in the form of graphite flakes. Gray cast iron is a brittle material, and its compressive strength is much higher than its tensile strength. The fracture surface of gray cast iron has a gray color, which is how it got its name.
Ductile Cast Iron (Nodular Cast Iron)
The addition of magnesium to gray cast iron improves the ductility of the material. The resulting material is called nodular cast iron because the magnesium causes the graphite flakes to form into spherical nodules. It is also called ductile cast iron. Nodular cast iron has good strength, ductility, and machinability. Common uses include crankshafts, gears, pump bodies, valves, and machine parts.
White Cast Iron
White cast iron has carbon in the form of carbide, which makes the material hard, brittle, and difficult to machine. White cast iron is primarily used for wear-resisting components as well as for the production of malleable cast iron.
Malleable Cast Iron
Malleable cast iron is produced by heat treating white cast iron. The heat treatment improves the ductility of the material while maintaining its high strength.
Aluminum Alloys
Pure aluminum is soft and weak, but it can be alloyed to increase strength. Pure aluminum has good corrosion resistance due to an oxide coating that forms over the material and prevents oxidation. Alloying the aluminum tends to reduce its corrosion resistance.
Aluminum is a widely used material, particularly in the aerospace industry, due to its light weight and corrosion resistance. Despite the fact that aluminum alloys are generally not as strong as steels, they nevertheless have a good strength-to-weight ratio.
Nickel Alloys
Nickel alloys have high temperature and corrosion resistance. Common alloying ingredients include copper, chromium, and iron. Common nickel alloys include Monel, K-Monel, Inconel, and Hastelloy
Copper Alloys
Copper alloys are generally characterized as being electrically conductive, having good corrosion resistance, and being relatively easy to form and cast. While they are a useful engineering material, copper alloys are also very attractive and are commonly used in decorative applications.
Copper alloys primarily consist of brasses and bronzes. Zinc is the major alloying ingredient in brass. Tin is a major alloying element in most bronzes. Bronzes may also contain aluminum, nickel, zinc, silicon, and other elements. The bronzes are typically stronger than the brasses while still maintaining good corrosion resistance.
Titanium Alloys
Titanium alloys are light, strong, and have high corrosion resistance. Their density is much lower than steel, and their strength-to-weight ratio is excellent. For this reason, titanium alloys are used fairly commonly, especially in the aerospace industry. One primary downside of titanium alloys is the high cost.
There are three categories of titanium alloys: alpha alloys, beta alloys, and alpha-beta alloys. Alpha alloys do not respond to heat treatment and are instead strengthened through solid-solution strengthening processes. The beta and alpha-beta alloys can be strengthened by heat treatment, primarily through precipitation hardening.
Q3) What are polymers?
A3)
Polymers
Polymers are materials that consist of molecules formed by long chains of repeating units. They may be natural or synthetic. Many useful engineering materials are polymers, such as plastics, rubbers, fibers, adhesives, and coatings. Polymers are classified as thermoplastic polymers, thermosetting polymers (thermosets), and elastomers.
Thermoplastic Polymers
The classification of thermoplastics and thermosets is based on their response to heat. If heat is applied to a thermoplastic, it will soften and melt. Once it is cooled, it will return to solid form. Thermoplastics do not experience any chemical change through repeated heating and cooling (unless the temperature is high enough to break the molecular bonds). They are therefore very well suited to injection molding.
Thermosetting Polymers
Thermosets are typically heated during initial processing, after which they become permanently hard. Thermosets will not melt upon reheating. If the applied heat becomes extreme however, the thermoset will degrade due to breaking of the molecular bonds. Thermosets typically have greater hardness and strength than thermoplastics. They also typically have better dimensional stability than thermoplastics, meaning that they are better at maintaining their original dimensions when subjected to temperature and moisture changes.
Elastomers
Elastomers are highly elastic polymers with mechanical properties similar to rubber. Elastomers are commonly used for seals, adhesives, hoses, belts, and other flexible parts. The strength and stiffness of rubber can be increased through a process called vulcanization, which involves adding sulfur and subjecting the material to high temperature and pressure. This process causes cross-links to form between the polymer chains.
Ceramics
Ceramics are solid compounds that may consist of metallic or nonmetallic elements. The primary classifications of ceramics include glasses, cements, clay products, refractories, and abrasives.
Ceramics generally have excellent corrosion and wear resistance, high melting temperature, high stiffness, and low electrical and thermal conductivity. Ceramics are also very brittle materials.
Glass
Glasses are common materials and are seen in applications including windows, lenses, and containers. Glasses are amorphous, whereas the other ceramics are mainly crystalline. Primary advantages of glasses include transparency and ease of fabrication. The base element of most glasses is silica, and other components can be added to modify its properties. Common processes used to form glass include:
- Heating until melting, then pouring into molds to cast into useful shapes
- Heating until soft, then rolling
- Heating until soft, then blowing into desired shapes
Cements
Cements are materials that, after mixing with water, form a paste that then hardens. Because of this characteristic, cements can be formed into useful shapes while in paste form before they harden into rigid structures. Plaster of Paris is one common cement. The most common cement is called Portland cement, which is made by mixing clay and limestone and then firing at high temperature. Portland cement is used to form concrete, which is made by mixing it with sand, gravel, and water. It can also be mixed with sand and water to form mortar. Like other ceramics, cements are weak in tension but strong in compression. Cement is very inexpensive to produce, and it is used widely in the construction of buildings, bridges, and other large structures.
Clay Products
Clay is a very common ceramic material. It can be mixed with water, shaped, and then hardened through firing at high temperature. The two primary classifications of clay products include structural clay products and whitewares. Structural clay products see applications including bricks, tiles, and piping. Whitewares see applications including pottery and plumbing fixtures.
Refractories
Refractory ceramics can withstand high temperatures and extreme environments. They can also provide thermal insulation. Brick is the most common refractory ceramic.
Abrasives
Abrasive ceramics are hard materials that are used to cut, grind, and wear away other softer materials. Typical properties of abrasives include high hardness, wear resistance, and temperature resistance. Abrasives can either be bonded to a surface (e.g., grinding wheels and sandpaper), or can be used as loose grains (e.g., sand blasting). Common abrasives include cemented carbide, silicon carbide, tungsten carbide, aluminum oxide, and silica sand. Diamond is also an excellent abrasive, but it is expensive.
Q4) Explain properties and applications of materials.
A4)
Mechanical properties of materials:
- Strength: Ability of material that can resist or withstand mechanical load.
- Ductility: Ability to material to form wires.
- Malleability: Ability of material to form sheets.
- Brittleness: Ability of a material to withstand mechanical load without plastic deformation.
- Hardness: Ability of a material that can offer resistance against mechanical deformation.
- Toughness: Ability of a material that can absorb energy at the time of failure.
- Stiffness: Ability of a material that can resist mechanical deformation under stress
- Resilience: Ability of a material that can absorb energy against failure, without undergoing shape change
Application:
- Atomic Resolution Microscopy: JEOL instrumentation is unequalled for atomic decision imaging. The ultrastable electron column and excessive decision of the ARM200F aberration-corrected TEM push substances studies to new frontiers. The unequalled uncooked facts from the ARM200F reveal never-before-visible imaging decision plus excessive spatial decision for chemical analysis. The ARM200F capabilities an all-new shielded electron column layout that surpasses all different TEM designs today.
- Biomaterials – Borrowing from: Nature Today’s substances technology researchers are main the manner in fabricating newer, more potent substances primarily based totally on those who arise in nature. Nacre, the iridescent, excellentb robust fabric referred to as mother-of-pearl, is sectioned the use of Focused Ion Beam (FIB) and studied the use of SEM for houses which could make a contribution to growing more potent substances at MIT’s Institute for Soldier Nanotechnologies. A flexible JEOL analytical, area emission TEM, the JEM-3200FS, is utilized in multidisciplinary research at Indiana University to increase self-assembled molecular layers and nanoparticles that mimic the self-meeting of viruses.
- Pioneering Nanotechnology :JEOL TEMs and SEMs have lengthy been used to advantage superior studies in addition to failure analysis. In the 1980s, a JEOL excessive decision TEM demonstrated the shape of C60 and helped result in early discoveries in carbon nanotubes. Nobel prize winners, recipients of fundamental studies grants, and world-famend scientists have used JEOL gadgets of their superior studies.
- Structural Imaging and Analysis: Microstructural records and surface/bulk chemical analyses are simply received from JEOL SEMs and Microprobes with ultra-modern results. Elemental mapping and characterization of high-quality systems are recurring for JEOL’s excessive overall performance FEG-SEMs, which includes the ultrahigh decision analytical area emission JSM-7600F. Coatings, adhesives, layers, composites – all is discovered through the electricity of the SEM and Microprobe.
- Nanofabrication: Versatile SEM/FIB tools, just like the JEOL MultiBeam, permit simultaneous viewing, analysis, and micro milling functions, and serial reducing and sampling (S3) for monitoring, reducing, fabrication and reconstruction of specimens in 3D. JEOL’s information in e-beam lithography expands the functionality of the Field Emission SEM to permit for direct write patterning and gas-assisted e-beam lithography. In the 1960s, direct write e-beam turned into used for writing IC circuits on small wafers for a committed application. Now it's miles utilized in a myriad of programs from photonics and DNA filters to nano-fluidics, nano-hole patterns, and unmarried electron transistors.
Q5) Explain crystalline nature of metals.
A5)
- Metal elements (besides Cs, Ga, and Hg) are crystalline solids at room temperature. Like ionic solids, metals and alloys have a totally sturdy tendency to crystallize, whether or not they may be made with the aid of using thermal processing or with the aid of using different strategies which include answer discount or electroplating.
- Metals crystallize without problems and it's far hard to shape a glassy metallic despite very speedy cooling. Molten metals have low viscosity, and the identical (basically spherical) atoms can % right into a crystal very easily. Glassy metals may be made, however, with the aid of using hastily cooling alloys, especially if the constituent atoms have one of a kind sizes.
- The one of a kind atoms cannot % in a easy unit cell, on occasion making crystallization sluggish sufficient to shape a glass. Most metals and alloys crystallize in certainly considered one among 3 very not unusualplace systems: body-focused cubic (bcc), hexagonal near packed (hcp), or cubic near packed (ccp, additionally known as face focused cubic, fcc).
- In all 3 systems the coordination quantity of the metallic atoms (i.e., the quantity of equidistant nearest associates) is alternatively high: eight for bcc, and 12 for hcp and ccp.
- We can evaluation this with the low coordination numbers (i.e., low valences - like 2 for O, three for N, or four for C) observed in nonmetals. In the bcc structure, the closest associates are on the corners of a dice surrounding the metallic atom withinside the center. In the hcp and ccp systems, the atoms % like stacked cannonballs or billiard balls, in layers with a six-coordinate arrangement.
- Each atom additionally has six extra nearest associates from layers above and below.
- The stacking series is ABCABC... With inside the ccp lattice and ABAB... In hcp. In each cases, it may be proven that the spheres fill 74% of the extent of the lattice. This is the best extent fraction that may be packed with a lattice of identical spheres.
- Atoms in steel crystals will be inclined to % in dense arrangments that fill area successfully. The easy rectangular packing (above) upon which the easy cubic shape is primarily based totally is inefficient and as a consequence uncommon amongst steel crystal systems. Body- or face-focused systems fill area greater successfully and greater common.
Q6) Write a note on specially microscopic and macroscopic examinations of metals.
A6)
- Metallography is the have a look at of the bodily shape and additives of metals, through the use of microscopy.
- Ceramic and polymeric substances can also be organized the use of metallographic techniques, therefore the phrases ceramography, plastography and, collectively, materialography.
- The floor of a metallographic specimen is ready through diverse techniques of grinding, sharpening, and etching. After coaching, it's far frequently analyzed the use of optical or electron microscopy.
- Using simplest metallographic techniques, a professional technician can perceive alloys and expect fabric properties. Mechanical coaching is the maximum not unusualplace coaching approach. Successively finer abrasive debris are used to put off fabric from the pattern floor till the favored floor exceptional is achieved. Many unique machines are to be had for doing this grinding and sharpening, which might be capable of meet unique needs for exceptional, capacity, and reproducibility.
- A systematic coaching approach is the perfect manner to obtain the authentic shape. Sample coaching should consequently pursue policies which might be appropriate for maximum substances. Different substances with comparable properties (hardness and ductility) will reply alike and accordingly require the equal consumables in the course of coaching.
- Metallographic specimens are typically "mounted" the use of a warm compression thermosetting resin. In the past, phenolic thermosetting resins were used, however current epoxy is turning into extra famous due to the fact decreased shrinkage in the course of curing outcomes in a higher mount with advanced part retention.
- A ordinary mounting cycle will compress the specimen and mounting media to 4,000 psi (28 MPa) and warmth to a temperature of 350 °F (177 °C). When specimens are very touchy to temperature, "bloodless mounts" can be made with a two-element epoxy resin.
- Mounting a specimen offers a safe, standardized, and ergonomic manner through which to keep a pattern in the course of the grinding and sharpening operations. A macro etched copper disc After mounting, the specimen is moist floor to show the floor of the metallic.
- The specimen is successively floor with finer and finer abrasive media. Silicon carbide abrasive paper became the primary approach of grinding and remains used today. Many metallographers, however, choose to use a diamond grit suspension that's dosed onto a reusable material pad during the sharpening process.
- Diamond grit in suspension may begin at nine micrometres and end at one micrometre. Generally, sharpening with diamond suspension offers finer outcomes than the use of silicon carbide papers (SiC papers), mainly with revealing porosity, which silicon carbide paper sometimes "smear" over. After grinding the specimen, sharpening is performed.
- Typically, a specimen is polished with a slurry of alumina, silica, or diamond on a napless fabric to supply a scratch-unfastened replicate end, unfastened from smear, drag, or pull-outs and with minimum deformation last from the coaching process.
- After sharpening, sure microstructural parts may be visible with the microscope, e.g., inclusions and nitrides. If the crystal shape is non-cubic (e.g., a metallic with a hexagonal-closed packed crystal shape, which include Ti or Zr) the microstructure may be discovered with out etching the use of crossed polarized mild (mild microscopy).
- Otherwise, the microstructural parts of the specimen are discovered through the use of a appropriate chemical or electrolytic etchant.
Q7) Write a short note on ferrous alloy.
A7)
Ferrous alloys:
Those in which iron is the primary component are produced in greater quantities than any other metal. They're particularly useful as engineering building materials. Three variables account for their extensive use: (1) iron-containing compounds are abundant in the earth's crust; (2) metallic iron and steel alloys can be made with relatively low-cost extraction, refining, alloying, and fabrication techniques; and (3) ferrous alloys are extremely versatile, as they can be tailored to have a wide range of mechanical and physical properties.
The main disadvantage of many ferrous alloys is their corrosion resistance. This section covers the compositions, microstructures, and properties of a variety of steels and cast irons.
Q8) What is steel?
A8)
Steels:
Steels are iron–carbon alloys that may contain significant amounts of additional alloying elements; there are thousands of distinct alloys with various compositions and heat treatments. The mechanical properties are affected by the carbon content, which is typically less than 1.0 wt%. Some of the more prevalent steels are divided into low-, medium-, and high-carbon categories based on carbon content. Within each group, subclasses exist based on the concentration of various alloying elements. Other than carbon and a small amount of manganese, plain carbon steels contain only residual impurities. More alloying elements are purposely introduced in specified concentrations to alloy steels.
1) Low carbon steel:
Low-carbon steels account for the majority of the numerous types of steel manufactured. These typically contain less than 0.25 wt% C and are insensitive to heat treatments designed to create martensite; cold work is used to reinforce them. The elements of microstructures are ferrite and pearlite. As a result, these alloys are relatively soft and weak but have exceptional ductility and toughness; they are also machinable, weldable, and the least expensive to create of all steels.
Automobile body components, structural shapes (I-beams, channel and angle iron), and sheets used in pipelines, buildings, bridges, and tin cans are only a few examples. They have a yield strength of 275 MPa (40,000 psi), tensile strengths of 415 to 550 MPa (60,000 to 80,000 psi), and 25 percent EL ductility. The high-strength, low-alloy (HSLA) steels are another type of low-carbon alloy. They have higher strengths than ordinary low-carbon steels because they incorporate various alloying elements such as copper, vanadium, nickel, and molybdenum in combined concentrations as high as 10% by weight.
Heat treatment can increase tensile strength to above 480 MPa (70,000 psi); they are also ductile, formable, and machinable. In normal environments, HSLA steels are more corrosion resistant than ordinary carbon steels, which they have largely supplanted in many applications where structural strength is required (for example, bridges, towers, high-rise building support columns, and pressure vessels).
2) Medium-Carbon Steels
Carbon contents in medium-carbon steels range from 0.25 to 0.60 wt percent. To improve their mechanical properties, these alloys can be heat-treated by austenitizing, quenching, and tempering. They are most used in tempered form, with tempered martensite microstructures. Plain medium-carbon steels have low hardenability and can only be heat-treated satisfactorily in very thin sections and at extremely high quenching rates.
Additions of chromium, nickel, and molybdenum improve the alloys' heat-treatability, resulting in a wide range of strength–ductility combinations. These heat-treated alloys are stronger than low-carbon steels, but ductility and toughness are sacrificed. Railway wheels and tracks, gears, crankshafts, and other machine elements, as well as high-strength structural components, all require a blend of strength, wear resistance, and toughness.
3) High-Carbon Steels
High-carbon steels, with carbon levels typically ranging from 0.60 to 1.4 wt%, are the hardest, strongest, and least ductile of the carbon steels. They are virtually always hardened and tempered, which makes them particularly wear resistant and capable of keeping a sharp cutting edge. High-carbon alloys with chromium, vanadium, tungsten, and molybdenum are used in tool and die steels. These alloying elements mix with carbon to generate carbide compounds that are extremely hard and wear resistant.
These steels are used in knives, razors, hacksaw blades, springs, and high-strength wire, as well as cutting tools and dies for moulding and shaping materials.
4) Stainless Steels
In a range of conditions, including the ambient atmosphere, stainless steels are highly resistant to corrosion (rusting). The most common alloying element is chromium, which must have a concentration of at least 11 weight percent Cr. Nickel and molybdenum additives can also improve corrosion resistance. On the basis of the major phase composition of the microstructure, stainless steels are classified as martensitic, ferritic, or austenitic. Stainless steels are extremely adaptable in their use due to their wide range of mechanical qualities and good corrosion resistance. Heat-treating martensitic stainless steels to make martensite the predominant microconstituent is possible. The iron–iron carbide phase diagram changes dramatically when alloying metals are added in considerable amounts.
Q9) What is cast iron?
A9)
Cast Iron
Cast irons are a type of ferrous alloy with a carbon concentration greater than 2.14 weight percent; however, most cast irons possess between 3.0 and 4.5 weight percent C, as well as other alloying elements. A closer look at the iron–iron carbide phase diagram reveals that alloys in this composition range become entirely liquid at temperatures between 1150 and 1300C (2100 and 2350F), which is significantly lower than steel temperatures. As a result, they are easily melted and castable. Furthermore, some cast irons are extremely fragile, and casting is the most practical method of manufacturing.
Gray iron
Gray cast irons have carbon and silicon concentrations of 2.5 to 4.0 wt percent and 1.0 to 3.0 wt percent, respectively. The graphite in most of these cast irons is in the form of flakes (like corn flakes), which are usually surrounded by an x-ferrite or pearlite matrix; this is the microstructure of a typical grey iron. A shattered surface takes on a grey look as a result of the graphite flakes, hence the name.
Solid solutions
When homogeneous mixtures of two or more kinds of atoms (of metal) occur in the solid state, they are known as the solid solutions.
The more abundant atomic form is referred as solvent and less abundant atomic form is referred as solute.
For example: - In sterling silver (92.5% of silver of remaining is copper) is a solid solution of silver and copper where silver is solvent and copper is solute.
Types of solid solutions: -
Solid solutions are generally of two types
1) Substitutional solid solutions.
2) Interstitial solid solutions
Substitutional solid solution:
As suggested by the name substitutional means 'replacement’. Here in this case when the atom of the parent metal or we can say atom of the solvent replaced by the atom of the solute metal then the solid solution is known as substitutional solid solution.
Hume Rothery rules for formation of substitutional solid solutions:
Hume Rothery rules defines us the various conditions under which an element court dissolve in a metal forming a solid solution.
These are as follows:
a) Crystal structure factor
b) Relative size factor
c) Chemical affinity sector
d) Relative valence factor
Crystal structure factor: - The crystal structure of solute and solvent must be similar. Example: - either crystal structure should be FCC, or BCC or HCP.
Relative size factor: - The atomic radius of the solute and solvent atoms must differ by not more than 15%. If it’s beyond 15% then it restricts solid solubility.
Chemical affinity factor: - When two metals have lesser chemical affinity then it forms solid solution. If there will be greater chemical affinity then it can cause compound formation. In others we can see that the solute and solvent should have similar electronegativity.
Relative valence factor: - The complete solubility occurs when solute and solvent must have same valency. A metal with lower valency is more likely to dissolve in metal of higher valency.
(2) Interstitial solid solutions: -
In interstitial solid solutions the solute atom does not displace a solvent atom but rather it enters one of the holes or interstices between the solvent atoms.
Q10) Explain types and their formations of steel.
A10)
- There are kinds of strong solutions: 1. Substitutional Solid Solution 2. Interstitial Solid Solutions. Solute is the minor detail this is delivered to the solvent, and solvent is the important detail of answer.
- When a specific crystal shape of the solvent is maintained at some point of alloying the alloy is referred to as a strong answer. The quantity of solute that can be dissolved through the solvent is commonly a feature of temperature (with strain constant) and normally growth with temperature. There are 3 feasible situation for answer i.e., unsaturated, saturated and supersaturated.
Type 1. Substitutional Solid Solution:
- If the scale of the solute atom is just like that of the solvent atom, the solute atoms can update solvent atoms to shape a substitutional stable solution. Example: Brass, wherein zinc (solute atom) is added into the lattice of copper (Solvent).
- Two situations are typically required to from entire substitutional stable solution:
(i) Two factors have to have comparable crystal structure.
(ii) The distinction of their atomic radii need to be much less than 15%. Example: In the Aluminium nickel alloy system, each steel are FCC. The relative length component is 14%. However, nickel is decrease in valence then Al and as in step with relative valence component stable nickel dissolve 5% Al however better valence Al, dissolve handiest 0.04 % Ni.
Type 2. Interstitial Solid Solutions:
- If the scale of the solute atom is plenty smaller than that of the solvent atom the solute atom can occupy an interstitial role forming interstitial stable solution. Since the distance of the lattice shape are confined in size, most effective atoms with atomic radii much less than 1 angstrom are usually shape interstitial stable solution.
- These are hydrogen (0.46), boron (0.97), Carbon (0.77), Nitrogen (0.71) and Oxygen (0.60). More solute atoms can be dissolved interstitially till the answer emerge as saturated at that temperature. Interstitial stable answers usually have constrained solubility and usually are of a touch importance. Carbon in iron is a exquisite exception and paperwork the premise for hardening steel. Carbon dissolves in iron interstitially.
- The most solubility of Carbon in iron (F.C.C.) is 2% at 1150°F at the same time as most solubility of carbon in α iron (B.C.C.) is most effective 0.025% at 727°C. Two important situations for forming ISS are: (i) The solvent atom ought to have a couple of valence. (ii) The atomic radius of solute atom ought to be much less than 59% of the atomic radii of solvent atom. Example: Steel, wherein carbon atoms are found in interstitial positions among iron atoms with most percent of 2. Atomic radius of carbon is 0.071 nm that is much less than 59% of 0.one hundred twenty five nm radius of iron atom.
Formation of solid solution:
- Stable answer, combination of crystalline solids that coexist as a brand new crystalline stable, or crystal lattice.
- The blending may be done through combining the 2 solids after they were melted into drinks at excessive temperatures after which cooling the end result to shape the brand new stable or through depositing vapours of the beginning substances onto substrates to shape skinny films. As with drinks, solids have one of a kind levels of mutual solubility, relying on their chemical residences and crystalline structure, which decide how their atoms suit collectively withinside the blended crystal lattice.
- The blended lattice can be substitutional, wherein the atoms of 1 beginning crystal update the ones of the other, or interstitial, wherein the atoms occupy positions commonly vacant withinside the lattice.
- The materials can be soluble over a partial or maybe whole variety of relative concentrations, generating a crystal whose residences range constantly over the variety.
- This presents a manner to tailor the residences of the stable answer for unique applications. Many stable answers seem in nature withinside the shape of minerals made below situations of warmth and pressure.
- One instance is the olivine mineral group, specially the forsterite-fayalite series, whose individuals range from forsterite (Mg2SiO4) to fayalite (Fe2SiO4).
- The compounds have same crystal systems and shape a substitutional stable answer that may variety from a hundred percentage magnesium (Mg) to a hundred percentage iron (Fe), along with all proportions in between, with bodily residences that change easily from the ones of forsterite to the ones of fayalite.
Q11) Explain modified Gibbs’s phase rule in detail.
A11)
- Gibbs segment rule states that if the equilibrium in a heterogeneous machine isn't stricken by gravity or through electric and magnetic forces, the wide variety of diploma of freedom is given through the equation
F=C-P+2
- Wherein C is the wide variety of chemical components P is the wide variety of levels Basically, it describes the mathematical dating for figuring out the steadiness of levels gift withinside the fabric at equilibrium condition. In the subsequent section, allow us to study the segment rule derivation.
- The Gibb’s section rule on the idea of the thermodynamic rule may be derived as follows: First, allow us to do not forget a heterogeneous device including Pn range of stages and Cn range of additives in equilibrium. Let us anticipate that the passage of a element from one section to any other doesn’t contain any chemical reaction.
- When the device is in equilibrium, it is able to be defined via way of means of the subsequent parameters: Temperature Pressure The composition of every section a. The general range of variables required to specify the nation of the device is: Pressure: identical for all stages Temperature: identical for all stages Concentration The impartial awareness variables for one section with admire to the C additives is C – 1. Therefore, the impartial awareness variables for P stages with admire to C additives is P (C – 1).
Total number of variables = P (C – 1) + 2 (1)
The total number of equilibria:
- The various phases present in the system can only remain in equilibrium when the chemical potential (µ) of each of the component is the same in all phases, i.e.
µ1, P1 = | µ1, P2 = | µ1, P3 = | … | = | µ1, P |
µ2, P1 = | µ2, P2 = | µ2, P3 = | … | = | µ2, P |
: | : | : |
|
| : |
: | : | : |
|
| : |
: | : | : |
|
| : |
µC, P1 = | µC, P2 = | µC, P3 = | … | = | µC, P |
The number of equilibria for each P phases for each component is P – 1.
For C components, the number of equilibria for P phases is P ( C – 1).
Hence, the total number of equilibria involved is E = C (P – 1). (2)
Equating eq (1) and (2), we get
F=[P(C−1)+2−C]−[C(P−1)]F=[P(C−1)+2−C]−[C(P−1)] F=[CP−P+2−CP+C]F=[CP−P+2−CP+C] F=C−P+2F=C−P+2
The obtained formula is the Gibbs phase rule. Stay tuned to BYJU’S to learn more physics derivations.
Q12) What is lever rule for phase mixtures and their application in system?
A12)
- If an alloy includes a couple of section, the quantity of every section gift may be located through making use of the lever rule to the section diagram. The lever rule may be defined through thinking about a easy stability.
- The composition of the alloy is represented through the fulcrum, and the compositions of the 2 levels through the ends of a bar. The proportions of the levels gift are decided through the weights had to stability the system.

Fig: Lever rule
So,
Fraction of phase 1 (C2 - C) / (C2 - C1)
And,
Fraction of phase 2 (C - C1) / (C2 - C1).
- Point 1 At factor 1 the alloy is absolutely liquid, with a composition C. Let C = sixty five weight% B.
- Point 2 At factor 2 the alloy has cooled as a ways because the liquidus, and strong section β begins offevolved to form. Phase β first bureaucracy with a composition of ninety six weight% B.
- The inexperienced dashed line underneath is an instance of a tie-line. A tie-line is a horizontal (i.e., constant-temperature) line via the selected factor, which intersects the section boundary strains on both side.
Q13) Explain study of equilibrium diagrams and invariant reactions.
A13)
- Almost all substances have a couple of section in them. Thus engineering substances obtain their unique properties. Macroscopic primary unit of a fabric is referred to as thing.
- It refers to a unbiased chemical species. The components of a machine can be elements, ions or compounds.
- A section may be described as a homogeneous part of a machine that has uniform bodily and chemical traits i.e. it's far a bodily wonderful from other phases, chemically homogeneous and mechanically separable part of a machine.
- A thing can exist in lots of phases. E.g.: Water exists as ice, liquid water, and water vapor. Carbon exists as graphite and diamond.
- When stages are found in a system, it isn't necessary that there be a distinction in each bodily and chemical homes; a disparity in a single or the alternative set of homes is sufficient.
- Answer (liquid or solid) is segment with greater than one aspect; a combination is a cloth with greater than one segment. Solute (minor aspect of in a answer) does not extrade the structural sample of the solvent, and the composition of any answer may be varied. In mixtures, there are one-of-a-kind stages, every with its own atomic arrangement. It is feasible to have a combination of one-of-a-kind solutions
Q14) Explain iron-Iron carbide equilibrium diagram
A14)
- Equilibrium means that adjustments happening in a device due to manner intending in a single course are completely compensated via way of means of adjustments because of the reversal of the manner withinside the device. So it's miles taken into consideration as a dynamic situation of stability among atomic moves wherein the consequent is zero.
- The charges of adjustments of temperature or of composition had been extraordinarily sluggish at some stage in the experimental work, in order that the alloy would “come to relaxation” earlier than a variable consisting of temperature, have been once more changed.
- The situation, therefore, is one in all relaxation in place of change. Equilibrium diagram suggest the following:
1.Temperature at which the stable alloy will begin melting and end melting.
2. Possible segment adjustments so as to arise because the end result of changing the composition or temperature.
- The diagram describes the best situations for 2 or greater stages to exist in equilibrium.
- For example, the water section diagram describes a point (triple point) wherein water can coexist in 3 special stages on the identical time. This takes place at simply above the freezing temperature (0.01°C) and 0.006 atm.
- Uses of Equilibrium Diagram in Metallurgy Development of the brand new alloys on the idea of software requirements.
- Production of those alloys. Development and implementation of suitable warmth remedy methods to enhance the chemical, physical, and mechanical homes of those new alloys.
- Troubleshooting problems that stand up all through using those new alloys, in the long run enhancing product predictability.
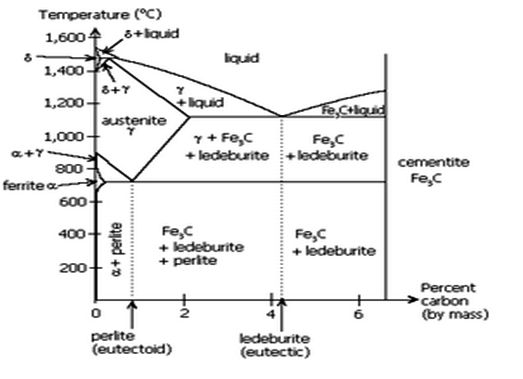
Fig: Iron-Iron carbide equilibrium diagram
- The Iron carbon equilibrium diagram (additionally known as the iron carbon section diagram) is a photograph illustration of the respective microstructure states of the alloy iron – carbon (Fe-C) relying on temperature and carbon content. The iron carbon section diagram is normally used to completely recognize the numerous stages of metal and forged iron.
- Steel and forged iron are each iron and carbon alloys. In addition, each alloys comprise hint factors in small amounts. The graph is pretty complex, however due to the fact we're restricting our research to Fe3C, we can handiest be searching at as much as 6.sixty seven weight percentage carbon.
Q15) What is critical temperature?
A15)
- Critical temperature of metal defines section transition among levels of metal. As the metal is heated above the essential temperature, approximately 1335°F (724°C), it undergoes a section change, recrystallizing as austenite.
- There are varieties of essential temperature: Lower essential temperature (Ac1). The temperature at which austenite begins offevolved to convert from ferrite. Upper essential temperature (Ac3).
- The temperature at which austenite is absolutely converted from ferrite. In the Fe-C system, there may be a eutectoid factor at about 0.8wt% C, 723°C. The section simply above the eutectoid temperature for undeniable carbon steels is called austenite or gamma.
- Steel grading structures don't forget chemical composition, treatment, and mechanical houses to permit fabricators to pick the best product for his or her application.
- Aside from the real percent of carbon and different alloys withinside the material, the microstructure additionally has a enormous affect at the mechanical houses of metal.
- It is vital to recognize the definition of microstructure—and the manner the microstructure of metal may be manipulated the use of warm and bloodless forming and after manufacturing.
- These strategies may be used to expand merchandise with unique mechanical houses. However, manipulating composition and microstructure will bring about a trade-off among one-of-a-kind houses.
- For example, more difficult metal can also additionally become with decreased strength. Microstructure The microstructure of a cloth is the manner wherein the molecules are joined collectively with forces at paintings among the ones molecules.
- Heating and cooling tactics are used to alternate the microstructure from one shape to another, thereby converting the houses of the material. Microstructure isn't observable to the bare eye however may be studied below a microscope. Steel can undertake numerous awesome microstructures—ferrite, pearlite, martensite, cementite, and austenite.
Unit - 1
Introduction to materials
Q1) What is material?
A1)
- Material choice is a totally essential step withinside the method of gadget layout. Selection of cloth relies upon following aspects: Performance Requirements: Material is chosen thinking about the layout constraints and overall performance necessities e.g. Hundreds performing at the member, size & weight constraints, environmental conditions, preferred reliability & sturdiness etc.
- Material Properties: Performance necessities are as compared with homes of substances to choose the nice appropriate cloth. For instance pressure degree expected for any gadget member is as compared with the power of the to be had substances.
- Properties may be Physical (melting point, co-green of thermal expansion, thermal conductivity, particular heat, particular gravity, electric conductivity, magnetic homes etc.), Chemical (corrosion resistance, reactivity with acids, bases, water etc.), Mechanical (hardness, toughness, ductility, malleability etc.) or Manufacturing (castability, weldability, formability, machineability etc.).
- Any of those homes can grow to be essential relying upon the layout necessities and environmental conditions.
- Manufacturing Aspects: Along with the choice of cloth fashion dressmaker additionally has to determine approximately the producing approaches for use to provide it preferred form.
- Therefore further to the producing homes of the cloth, production constraints also are to be taken care of, even as choosing a selected cloth.
- Availability & Cost: Material decided on need to be without difficulty to be had at a suitable fee. In addition to the cloth fee, general fee of fabrication is likewise taken into consideration because the preferred form has to receive to the cloth with nice pleasant and least fee. Availability of a massive quantity of substances with various homes makes the activity of cloth choice very difficult.
Q2) Classify the metal in detail.
A2)
Engineering materials refers to the group of materials that are used in the construction of manmade structures and components. The primary function of an engineering material is to withstand applied loading without breaking and without exhibiting excessive deflection. The major classifications of engineering materials include metals, polymers, ceramics, and composites. The important characteristics of the materials within each of these classes are discussed on this page, and tables of material properties are also provided.
Metals
Metals are the most commonly used class of engineering material. Metal alloys are especially common, and they are formed by combining a metal with one or more other metallic and/or non-metallic materials. The combination usually occurs through a process of melting, mixing, and cooling. The goal of alloying is to improve the properties of the base material in some desirable way.
Metal alloy compositions are described in terms of the percentages of the various elements in the alloy, where the percentages are measured by weight.
Ferrous Alloys
Ferrous alloys have iron as the base element. These alloys and include steels and cast irons. Ferrous alloys are the most common metal alloys in use due to the abundance of iron, ease of production, and high versatility of the material. The biggest disadvantage of many ferrous alloys is low corrosion resistance.
Carbon is an important alloying element in all ferrous alloys. In general, higher levels of carbon increase strength and hardness, and decrease ductility and weldability.
Carbon Steel
Carbon steels are basically just mixtures of iron and carbon. They may contain small amounts of other elements, but carbon is the primary alloying ingredient. The effect of adding carbon is an increase in strength and hardness.
Most carbon steels are plain carbon steels, of which there are several types.
Low-Alloy Steel
Low-alloy steels, also commonly called alloy steels, contain less than about 8% total alloying ingredients. Low-alloy steels are typically stronger than carbon steels and have better corrosion resistance.
Some low-alloy steels are designated as high-strength low-alloy (HSLA) steels. What sets HSLA steels apart from other low-alloy steels is that they are designed to achieve specific mechanical properties rather than to meet a specific chemical composition
Tool Steel
Tool steels are primarily used to make tooling for use in manufacturing, for example cutting tools, drill bits, punches, dies, and chisels. Alloying elements are typically chosen to optimize hardness, wear resistance, and toughness.
Stainless Steel
Stainless steels have good corrosion resistance, mostly due to the addition of chromium as an alloying ingredient. Stainless steels have a chromium composition of at least 11%. Passivation occurs with chromium content at or above 12%, in which case a protective inert film of chromic oxide forms over the material and prevents oxidation. The corrosion resistance of stainless steel is a result of this passivation.
Cast Iron
Cast iron is a ferrous alloy containing high levels of carbon, generally greater than 2%. The carbon present in the cast iron can take the form of graphite or carbide. Cast irons have a low melting temperature which makes them well suited to casting.
Gray Cast Iron
Gray cast iron is the most common type. The carbon is in the form of graphite flakes. Gray cast iron is a brittle material, and its compressive strength is much higher than its tensile strength. The fracture surface of gray cast iron has a gray color, which is how it got its name.
Ductile Cast Iron (Nodular Cast Iron)
The addition of magnesium to gray cast iron improves the ductility of the material. The resulting material is called nodular cast iron because the magnesium causes the graphite flakes to form into spherical nodules. It is also called ductile cast iron. Nodular cast iron has good strength, ductility, and machinability. Common uses include crankshafts, gears, pump bodies, valves, and machine parts.
White Cast Iron
White cast iron has carbon in the form of carbide, which makes the material hard, brittle, and difficult to machine. White cast iron is primarily used for wear-resisting components as well as for the production of malleable cast iron.
Malleable Cast Iron
Malleable cast iron is produced by heat treating white cast iron. The heat treatment improves the ductility of the material while maintaining its high strength.
Aluminum Alloys
Pure aluminum is soft and weak, but it can be alloyed to increase strength. Pure aluminum has good corrosion resistance due to an oxide coating that forms over the material and prevents oxidation. Alloying the aluminum tends to reduce its corrosion resistance.
Aluminum is a widely used material, particularly in the aerospace industry, due to its light weight and corrosion resistance. Despite the fact that aluminum alloys are generally not as strong as steels, they nevertheless have a good strength-to-weight ratio.
Nickel Alloys
Nickel alloys have high temperature and corrosion resistance. Common alloying ingredients include copper, chromium, and iron. Common nickel alloys include Monel, K-Monel, Inconel, and Hastelloy
Copper Alloys
Copper alloys are generally characterized as being electrically conductive, having good corrosion resistance, and being relatively easy to form and cast. While they are a useful engineering material, copper alloys are also very attractive and are commonly used in decorative applications.
Copper alloys primarily consist of brasses and bronzes. Zinc is the major alloying ingredient in brass. Tin is a major alloying element in most bronzes. Bronzes may also contain aluminum, nickel, zinc, silicon, and other elements. The bronzes are typically stronger than the brasses while still maintaining good corrosion resistance.
Titanium Alloys
Titanium alloys are light, strong, and have high corrosion resistance. Their density is much lower than steel, and their strength-to-weight ratio is excellent. For this reason, titanium alloys are used fairly commonly, especially in the aerospace industry. One primary downside of titanium alloys is the high cost.
There are three categories of titanium alloys: alpha alloys, beta alloys, and alpha-beta alloys. Alpha alloys do not respond to heat treatment and are instead strengthened through solid-solution strengthening processes. The beta and alpha-beta alloys can be strengthened by heat treatment, primarily through precipitation hardening.
Q3) What are polymers?
A3)
Polymers
Polymers are materials that consist of molecules formed by long chains of repeating units. They may be natural or synthetic. Many useful engineering materials are polymers, such as plastics, rubbers, fibers, adhesives, and coatings. Polymers are classified as thermoplastic polymers, thermosetting polymers (thermosets), and elastomers.
Thermoplastic Polymers
The classification of thermoplastics and thermosets is based on their response to heat. If heat is applied to a thermoplastic, it will soften and melt. Once it is cooled, it will return to solid form. Thermoplastics do not experience any chemical change through repeated heating and cooling (unless the temperature is high enough to break the molecular bonds). They are therefore very well suited to injection molding.
Thermosetting Polymers
Thermosets are typically heated during initial processing, after which they become permanently hard. Thermosets will not melt upon reheating. If the applied heat becomes extreme however, the thermoset will degrade due to breaking of the molecular bonds. Thermosets typically have greater hardness and strength than thermoplastics. They also typically have better dimensional stability than thermoplastics, meaning that they are better at maintaining their original dimensions when subjected to temperature and moisture changes.
Elastomers
Elastomers are highly elastic polymers with mechanical properties similar to rubber. Elastomers are commonly used for seals, adhesives, hoses, belts, and other flexible parts. The strength and stiffness of rubber can be increased through a process called vulcanization, which involves adding sulfur and subjecting the material to high temperature and pressure. This process causes cross-links to form between the polymer chains.
Ceramics
Ceramics are solid compounds that may consist of metallic or nonmetallic elements. The primary classifications of ceramics include glasses, cements, clay products, refractories, and abrasives.
Ceramics generally have excellent corrosion and wear resistance, high melting temperature, high stiffness, and low electrical and thermal conductivity. Ceramics are also very brittle materials.
Glass
Glasses are common materials and are seen in applications including windows, lenses, and containers. Glasses are amorphous, whereas the other ceramics are mainly crystalline. Primary advantages of glasses include transparency and ease of fabrication. The base element of most glasses is silica, and other components can be added to modify its properties. Common processes used to form glass include:
- Heating until melting, then pouring into molds to cast into useful shapes
- Heating until soft, then rolling
- Heating until soft, then blowing into desired shapes
Cements
Cements are materials that, after mixing with water, form a paste that then hardens. Because of this characteristic, cements can be formed into useful shapes while in paste form before they harden into rigid structures. Plaster of Paris is one common cement. The most common cement is called Portland cement, which is made by mixing clay and limestone and then firing at high temperature. Portland cement is used to form concrete, which is made by mixing it with sand, gravel, and water. It can also be mixed with sand and water to form mortar. Like other ceramics, cements are weak in tension but strong in compression. Cement is very inexpensive to produce, and it is used widely in the construction of buildings, bridges, and other large structures.
Clay Products
Clay is a very common ceramic material. It can be mixed with water, shaped, and then hardened through firing at high temperature. The two primary classifications of clay products include structural clay products and whitewares. Structural clay products see applications including bricks, tiles, and piping. Whitewares see applications including pottery and plumbing fixtures.
Refractories
Refractory ceramics can withstand high temperatures and extreme environments. They can also provide thermal insulation. Brick is the most common refractory ceramic.
Abrasives
Abrasive ceramics are hard materials that are used to cut, grind, and wear away other softer materials. Typical properties of abrasives include high hardness, wear resistance, and temperature resistance. Abrasives can either be bonded to a surface (e.g., grinding wheels and sandpaper), or can be used as loose grains (e.g., sand blasting). Common abrasives include cemented carbide, silicon carbide, tungsten carbide, aluminum oxide, and silica sand. Diamond is also an excellent abrasive, but it is expensive.
Q4) Explain properties and applications of materials.
A4)
Mechanical properties of materials:
- Strength: Ability of material that can resist or withstand mechanical load.
- Ductility: Ability to material to form wires.
- Malleability: Ability of material to form sheets.
- Brittleness: Ability of a material to withstand mechanical load without plastic deformation.
- Hardness: Ability of a material that can offer resistance against mechanical deformation.
- Toughness: Ability of a material that can absorb energy at the time of failure.
- Stiffness: Ability of a material that can resist mechanical deformation under stress
- Resilience: Ability of a material that can absorb energy against failure, without undergoing shape change
Application:
- Atomic Resolution Microscopy: JEOL instrumentation is unequalled for atomic decision imaging. The ultrastable electron column and excessive decision of the ARM200F aberration-corrected TEM push substances studies to new frontiers. The unequalled uncooked facts from the ARM200F reveal never-before-visible imaging decision plus excessive spatial decision for chemical analysis. The ARM200F capabilities an all-new shielded electron column layout that surpasses all different TEM designs today.
- Biomaterials – Borrowing from: Nature Today’s substances technology researchers are main the manner in fabricating newer, more potent substances primarily based totally on those who arise in nature. Nacre, the iridescent, excellentb robust fabric referred to as mother-of-pearl, is sectioned the use of Focused Ion Beam (FIB) and studied the use of SEM for houses which could make a contribution to growing more potent substances at MIT’s Institute for Soldier Nanotechnologies. A flexible JEOL analytical, area emission TEM, the JEM-3200FS, is utilized in multidisciplinary research at Indiana University to increase self-assembled molecular layers and nanoparticles that mimic the self-meeting of viruses.
- Pioneering Nanotechnology :JEOL TEMs and SEMs have lengthy been used to advantage superior studies in addition to failure analysis. In the 1980s, a JEOL excessive decision TEM demonstrated the shape of C60 and helped result in early discoveries in carbon nanotubes. Nobel prize winners, recipients of fundamental studies grants, and world-famend scientists have used JEOL gadgets of their superior studies.
- Structural Imaging and Analysis: Microstructural records and surface/bulk chemical analyses are simply received from JEOL SEMs and Microprobes with ultra-modern results. Elemental mapping and characterization of high-quality systems are recurring for JEOL’s excessive overall performance FEG-SEMs, which includes the ultrahigh decision analytical area emission JSM-7600F. Coatings, adhesives, layers, composites – all is discovered through the electricity of the SEM and Microprobe.
- Nanofabrication: Versatile SEM/FIB tools, just like the JEOL MultiBeam, permit simultaneous viewing, analysis, and micro milling functions, and serial reducing and sampling (S3) for monitoring, reducing, fabrication and reconstruction of specimens in 3D. JEOL’s information in e-beam lithography expands the functionality of the Field Emission SEM to permit for direct write patterning and gas-assisted e-beam lithography. In the 1960s, direct write e-beam turned into used for writing IC circuits on small wafers for a committed application. Now it's miles utilized in a myriad of programs from photonics and DNA filters to nano-fluidics, nano-hole patterns, and unmarried electron transistors.
Q5) Explain crystalline nature of metals.
A5)
- Metal elements (besides Cs, Ga, and Hg) are crystalline solids at room temperature. Like ionic solids, metals and alloys have a totally sturdy tendency to crystallize, whether or not they may be made with the aid of using thermal processing or with the aid of using different strategies which include answer discount or electroplating.
- Metals crystallize without problems and it's far hard to shape a glassy metallic despite very speedy cooling. Molten metals have low viscosity, and the identical (basically spherical) atoms can % right into a crystal very easily. Glassy metals may be made, however, with the aid of using hastily cooling alloys, especially if the constituent atoms have one of a kind sizes.
- The one of a kind atoms cannot % in a easy unit cell, on occasion making crystallization sluggish sufficient to shape a glass. Most metals and alloys crystallize in certainly considered one among 3 very not unusualplace systems: body-focused cubic (bcc), hexagonal near packed (hcp), or cubic near packed (ccp, additionally known as face focused cubic, fcc).
- In all 3 systems the coordination quantity of the metallic atoms (i.e., the quantity of equidistant nearest associates) is alternatively high: eight for bcc, and 12 for hcp and ccp.
- We can evaluation this with the low coordination numbers (i.e., low valences - like 2 for O, three for N, or four for C) observed in nonmetals. In the bcc structure, the closest associates are on the corners of a dice surrounding the metallic atom withinside the center. In the hcp and ccp systems, the atoms % like stacked cannonballs or billiard balls, in layers with a six-coordinate arrangement.
- Each atom additionally has six extra nearest associates from layers above and below.
- The stacking series is ABCABC... With inside the ccp lattice and ABAB... In hcp. In each cases, it may be proven that the spheres fill 74% of the extent of the lattice. This is the best extent fraction that may be packed with a lattice of identical spheres.
- Atoms in steel crystals will be inclined to % in dense arrangments that fill area successfully. The easy rectangular packing (above) upon which the easy cubic shape is primarily based totally is inefficient and as a consequence uncommon amongst steel crystal systems. Body- or face-focused systems fill area greater successfully and greater common.
Q6) Write a note on specially microscopic and macroscopic examinations of metals.
A6)
- Metallography is the have a look at of the bodily shape and additives of metals, through the use of microscopy.
- Ceramic and polymeric substances can also be organized the use of metallographic techniques, therefore the phrases ceramography, plastography and, collectively, materialography.
- The floor of a metallographic specimen is ready through diverse techniques of grinding, sharpening, and etching. After coaching, it's far frequently analyzed the use of optical or electron microscopy.
- Using simplest metallographic techniques, a professional technician can perceive alloys and expect fabric properties. Mechanical coaching is the maximum not unusualplace coaching approach. Successively finer abrasive debris are used to put off fabric from the pattern floor till the favored floor exceptional is achieved. Many unique machines are to be had for doing this grinding and sharpening, which might be capable of meet unique needs for exceptional, capacity, and reproducibility.
- A systematic coaching approach is the perfect manner to obtain the authentic shape. Sample coaching should consequently pursue policies which might be appropriate for maximum substances. Different substances with comparable properties (hardness and ductility) will reply alike and accordingly require the equal consumables in the course of coaching.
- Metallographic specimens are typically "mounted" the use of a warm compression thermosetting resin. In the past, phenolic thermosetting resins were used, however current epoxy is turning into extra famous due to the fact decreased shrinkage in the course of curing outcomes in a higher mount with advanced part retention.
- A ordinary mounting cycle will compress the specimen and mounting media to 4,000 psi (28 MPa) and warmth to a temperature of 350 °F (177 °C). When specimens are very touchy to temperature, "bloodless mounts" can be made with a two-element epoxy resin.
- Mounting a specimen offers a safe, standardized, and ergonomic manner through which to keep a pattern in the course of the grinding and sharpening operations. A macro etched copper disc After mounting, the specimen is moist floor to show the floor of the metallic.
- The specimen is successively floor with finer and finer abrasive media. Silicon carbide abrasive paper became the primary approach of grinding and remains used today. Many metallographers, however, choose to use a diamond grit suspension that's dosed onto a reusable material pad during the sharpening process.
- Diamond grit in suspension may begin at nine micrometres and end at one micrometre. Generally, sharpening with diamond suspension offers finer outcomes than the use of silicon carbide papers (SiC papers), mainly with revealing porosity, which silicon carbide paper sometimes "smear" over. After grinding the specimen, sharpening is performed.
- Typically, a specimen is polished with a slurry of alumina, silica, or diamond on a napless fabric to supply a scratch-unfastened replicate end, unfastened from smear, drag, or pull-outs and with minimum deformation last from the coaching process.
- After sharpening, sure microstructural parts may be visible with the microscope, e.g., inclusions and nitrides. If the crystal shape is non-cubic (e.g., a metallic with a hexagonal-closed packed crystal shape, which include Ti or Zr) the microstructure may be discovered with out etching the use of crossed polarized mild (mild microscopy).
- Otherwise, the microstructural parts of the specimen are discovered through the use of a appropriate chemical or electrolytic etchant.
Q7) Write a short note on ferrous alloy.
A7)
Ferrous alloys:
Those in which iron is the primary component are produced in greater quantities than any other metal. They're particularly useful as engineering building materials. Three variables account for their extensive use: (1) iron-containing compounds are abundant in the earth's crust; (2) metallic iron and steel alloys can be made with relatively low-cost extraction, refining, alloying, and fabrication techniques; and (3) ferrous alloys are extremely versatile, as they can be tailored to have a wide range of mechanical and physical properties.
The main disadvantage of many ferrous alloys is their corrosion resistance. This section covers the compositions, microstructures, and properties of a variety of steels and cast irons.
Q8) What is steel?
A8)
Steels:
Steels are iron–carbon alloys that may contain significant amounts of additional alloying elements; there are thousands of distinct alloys with various compositions and heat treatments. The mechanical properties are affected by the carbon content, which is typically less than 1.0 wt%. Some of the more prevalent steels are divided into low-, medium-, and high-carbon categories based on carbon content. Within each group, subclasses exist based on the concentration of various alloying elements. Other than carbon and a small amount of manganese, plain carbon steels contain only residual impurities. More alloying elements are purposely introduced in specified concentrations to alloy steels.
1) Low carbon steel:
Low-carbon steels account for the majority of the numerous types of steel manufactured. These typically contain less than 0.25 wt% C and are insensitive to heat treatments designed to create martensite; cold work is used to reinforce them. The elements of microstructures are ferrite and pearlite. As a result, these alloys are relatively soft and weak but have exceptional ductility and toughness; they are also machinable, weldable, and the least expensive to create of all steels.
Automobile body components, structural shapes (I-beams, channel and angle iron), and sheets used in pipelines, buildings, bridges, and tin cans are only a few examples. They have a yield strength of 275 MPa (40,000 psi), tensile strengths of 415 to 550 MPa (60,000 to 80,000 psi), and 25 percent EL ductility. The high-strength, low-alloy (HSLA) steels are another type of low-carbon alloy. They have higher strengths than ordinary low-carbon steels because they incorporate various alloying elements such as copper, vanadium, nickel, and molybdenum in combined concentrations as high as 10% by weight.
Heat treatment can increase tensile strength to above 480 MPa (70,000 psi); they are also ductile, formable, and machinable. In normal environments, HSLA steels are more corrosion resistant than ordinary carbon steels, which they have largely supplanted in many applications where structural strength is required (for example, bridges, towers, high-rise building support columns, and pressure vessels).
2) Medium-Carbon Steels
Carbon contents in medium-carbon steels range from 0.25 to 0.60 wt percent. To improve their mechanical properties, these alloys can be heat-treated by austenitizing, quenching, and tempering. They are most used in tempered form, with tempered martensite microstructures. Plain medium-carbon steels have low hardenability and can only be heat-treated satisfactorily in very thin sections and at extremely high quenching rates.
Additions of chromium, nickel, and molybdenum improve the alloys' heat-treatability, resulting in a wide range of strength–ductility combinations. These heat-treated alloys are stronger than low-carbon steels, but ductility and toughness are sacrificed. Railway wheels and tracks, gears, crankshafts, and other machine elements, as well as high-strength structural components, all require a blend of strength, wear resistance, and toughness.
3) High-Carbon Steels
High-carbon steels, with carbon levels typically ranging from 0.60 to 1.4 wt%, are the hardest, strongest, and least ductile of the carbon steels. They are virtually always hardened and tempered, which makes them particularly wear resistant and capable of keeping a sharp cutting edge. High-carbon alloys with chromium, vanadium, tungsten, and molybdenum are used in tool and die steels. These alloying elements mix with carbon to generate carbide compounds that are extremely hard and wear resistant.
These steels are used in knives, razors, hacksaw blades, springs, and high-strength wire, as well as cutting tools and dies for moulding and shaping materials.
4) Stainless Steels
In a range of conditions, including the ambient atmosphere, stainless steels are highly resistant to corrosion (rusting). The most common alloying element is chromium, which must have a concentration of at least 11 weight percent Cr. Nickel and molybdenum additives can also improve corrosion resistance. On the basis of the major phase composition of the microstructure, stainless steels are classified as martensitic, ferritic, or austenitic. Stainless steels are extremely adaptable in their use due to their wide range of mechanical qualities and good corrosion resistance. Heat-treating martensitic stainless steels to make martensite the predominant microconstituent is possible. The iron–iron carbide phase diagram changes dramatically when alloying metals are added in considerable amounts.
Q9) What is cast iron?
A9)
Cast Iron
Cast irons are a type of ferrous alloy with a carbon concentration greater than 2.14 weight percent; however, most cast irons possess between 3.0 and 4.5 weight percent C, as well as other alloying elements. A closer look at the iron–iron carbide phase diagram reveals that alloys in this composition range become entirely liquid at temperatures between 1150 and 1300C (2100 and 2350F), which is significantly lower than steel temperatures. As a result, they are easily melted and castable. Furthermore, some cast irons are extremely fragile, and casting is the most practical method of manufacturing.
Gray iron
Gray cast irons have carbon and silicon concentrations of 2.5 to 4.0 wt percent and 1.0 to 3.0 wt percent, respectively. The graphite in most of these cast irons is in the form of flakes (like corn flakes), which are usually surrounded by an x-ferrite or pearlite matrix; this is the microstructure of a typical grey iron. A shattered surface takes on a grey look as a result of the graphite flakes, hence the name.
Solid solutions
When homogeneous mixtures of two or more kinds of atoms (of metal) occur in the solid state, they are known as the solid solutions.
The more abundant atomic form is referred as solvent and less abundant atomic form is referred as solute.
For example: - In sterling silver (92.5% of silver of remaining is copper) is a solid solution of silver and copper where silver is solvent and copper is solute.
Types of solid solutions: -
Solid solutions are generally of two types
1) Substitutional solid solutions.
2) Interstitial solid solutions
Substitutional solid solution:
As suggested by the name substitutional means 'replacement’. Here in this case when the atom of the parent metal or we can say atom of the solvent replaced by the atom of the solute metal then the solid solution is known as substitutional solid solution.
Hume Rothery rules for formation of substitutional solid solutions:
Hume Rothery rules defines us the various conditions under which an element court dissolve in a metal forming a solid solution.
These are as follows:
a) Crystal structure factor
b) Relative size factor
c) Chemical affinity sector
d) Relative valence factor
Crystal structure factor: - The crystal structure of solute and solvent must be similar. Example: - either crystal structure should be FCC, or BCC or HCP.
Relative size factor: - The atomic radius of the solute and solvent atoms must differ by not more than 15%. If it’s beyond 15% then it restricts solid solubility.
Chemical affinity factor: - When two metals have lesser chemical affinity then it forms solid solution. If there will be greater chemical affinity then it can cause compound formation. In others we can see that the solute and solvent should have similar electronegativity.
Relative valence factor: - The complete solubility occurs when solute and solvent must have same valency. A metal with lower valency is more likely to dissolve in metal of higher valency.
(2) Interstitial solid solutions: -
In interstitial solid solutions the solute atom does not displace a solvent atom but rather it enters one of the holes or interstices between the solvent atoms.
Q10) Explain types and their formations of steel.
A10)
- There are kinds of strong solutions: 1. Substitutional Solid Solution 2. Interstitial Solid Solutions. Solute is the minor detail this is delivered to the solvent, and solvent is the important detail of answer.
- When a specific crystal shape of the solvent is maintained at some point of alloying the alloy is referred to as a strong answer. The quantity of solute that can be dissolved through the solvent is commonly a feature of temperature (with strain constant) and normally growth with temperature. There are 3 feasible situation for answer i.e., unsaturated, saturated and supersaturated.
Type 1. Substitutional Solid Solution:
- If the scale of the solute atom is just like that of the solvent atom, the solute atoms can update solvent atoms to shape a substitutional stable solution. Example: Brass, wherein zinc (solute atom) is added into the lattice of copper (Solvent).
- Two situations are typically required to from entire substitutional stable solution:
(i) Two factors have to have comparable crystal structure.
(ii) The distinction of their atomic radii need to be much less than 15%. Example: In the Aluminium nickel alloy system, each steel are FCC. The relative length component is 14%. However, nickel is decrease in valence then Al and as in step with relative valence component stable nickel dissolve 5% Al however better valence Al, dissolve handiest 0.04 % Ni.
Type 2. Interstitial Solid Solutions:
- If the scale of the solute atom is plenty smaller than that of the solvent atom the solute atom can occupy an interstitial role forming interstitial stable solution. Since the distance of the lattice shape are confined in size, most effective atoms with atomic radii much less than 1 angstrom are usually shape interstitial stable solution.
- These are hydrogen (0.46), boron (0.97), Carbon (0.77), Nitrogen (0.71) and Oxygen (0.60). More solute atoms can be dissolved interstitially till the answer emerge as saturated at that temperature. Interstitial stable answers usually have constrained solubility and usually are of a touch importance. Carbon in iron is a exquisite exception and paperwork the premise for hardening steel. Carbon dissolves in iron interstitially.
- The most solubility of Carbon in iron (F.C.C.) is 2% at 1150°F at the same time as most solubility of carbon in α iron (B.C.C.) is most effective 0.025% at 727°C. Two important situations for forming ISS are: (i) The solvent atom ought to have a couple of valence. (ii) The atomic radius of solute atom ought to be much less than 59% of the atomic radii of solvent atom. Example: Steel, wherein carbon atoms are found in interstitial positions among iron atoms with most percent of 2. Atomic radius of carbon is 0.071 nm that is much less than 59% of 0.one hundred twenty five nm radius of iron atom.
Formation of solid solution:
- Stable answer, combination of crystalline solids that coexist as a brand new crystalline stable, or crystal lattice.
- The blending may be done through combining the 2 solids after they were melted into drinks at excessive temperatures after which cooling the end result to shape the brand new stable or through depositing vapours of the beginning substances onto substrates to shape skinny films. As with drinks, solids have one of a kind levels of mutual solubility, relying on their chemical residences and crystalline structure, which decide how their atoms suit collectively withinside the blended crystal lattice.
- The blended lattice can be substitutional, wherein the atoms of 1 beginning crystal update the ones of the other, or interstitial, wherein the atoms occupy positions commonly vacant withinside the lattice.
- The materials can be soluble over a partial or maybe whole variety of relative concentrations, generating a crystal whose residences range constantly over the variety.
- This presents a manner to tailor the residences of the stable answer for unique applications. Many stable answers seem in nature withinside the shape of minerals made below situations of warmth and pressure.
- One instance is the olivine mineral group, specially the forsterite-fayalite series, whose individuals range from forsterite (Mg2SiO4) to fayalite (Fe2SiO4).
- The compounds have same crystal systems and shape a substitutional stable answer that may variety from a hundred percentage magnesium (Mg) to a hundred percentage iron (Fe), along with all proportions in between, with bodily residences that change easily from the ones of forsterite to the ones of fayalite.
Q11) Explain modified Gibbs’s phase rule in detail.
A11)
- Gibbs segment rule states that if the equilibrium in a heterogeneous machine isn't stricken by gravity or through electric and magnetic forces, the wide variety of diploma of freedom is given through the equation
F=C-P+2
- Wherein C is the wide variety of chemical components P is the wide variety of levels Basically, it describes the mathematical dating for figuring out the steadiness of levels gift withinside the fabric at equilibrium condition. In the subsequent section, allow us to study the segment rule derivation.
- The Gibb’s section rule on the idea of the thermodynamic rule may be derived as follows: First, allow us to do not forget a heterogeneous device including Pn range of stages and Cn range of additives in equilibrium. Let us anticipate that the passage of a element from one section to any other doesn’t contain any chemical reaction.
- When the device is in equilibrium, it is able to be defined via way of means of the subsequent parameters: Temperature Pressure The composition of every section a. The general range of variables required to specify the nation of the device is: Pressure: identical for all stages Temperature: identical for all stages Concentration The impartial awareness variables for one section with admire to the C additives is C – 1. Therefore, the impartial awareness variables for P stages with admire to C additives is P (C – 1).
Total number of variables = P (C – 1) + 2 (1)
The total number of equilibria:
- The various phases present in the system can only remain in equilibrium when the chemical potential (µ) of each of the component is the same in all phases, i.e.
µ1, P1 = | µ1, P2 = | µ1, P3 = | … | = | µ1, P |
µ2, P1 = | µ2, P2 = | µ2, P3 = | … | = | µ2, P |
: | : | : |
|
| : |
: | : | : |
|
| : |
: | : | : |
|
| : |
µC, P1 = | µC, P2 = | µC, P3 = | … | = | µC, P |
The number of equilibria for each P phases for each component is P – 1.
For C components, the number of equilibria for P phases is P ( C – 1).
Hence, the total number of equilibria involved is E = C (P – 1). (2)
Equating eq (1) and (2), we get
F=[P(C−1)+2−C]−[C(P−1)]F=[P(C−1)+2−C]−[C(P−1)] F=[CP−P+2−CP+C]F=[CP−P+2−CP+C] F=C−P+2F=C−P+2
The obtained formula is the Gibbs phase rule. Stay tuned to BYJU’S to learn more physics derivations.
Q12) What is lever rule for phase mixtures and their application in system?
A12)
- If an alloy includes a couple of section, the quantity of every section gift may be located through making use of the lever rule to the section diagram. The lever rule may be defined through thinking about a easy stability.
- The composition of the alloy is represented through the fulcrum, and the compositions of the 2 levels through the ends of a bar. The proportions of the levels gift are decided through the weights had to stability the system.

Fig: Lever rule
So,
Fraction of phase 1 (C2 - C) / (C2 - C1)
And,
Fraction of phase 2 (C - C1) / (C2 - C1).
- Point 1 At factor 1 the alloy is absolutely liquid, with a composition C. Let C = sixty five weight% B.
- Point 2 At factor 2 the alloy has cooled as a ways because the liquidus, and strong section β begins offevolved to form. Phase β first bureaucracy with a composition of ninety six weight% B.
- The inexperienced dashed line underneath is an instance of a tie-line. A tie-line is a horizontal (i.e., constant-temperature) line via the selected factor, which intersects the section boundary strains on both side.
Q13) Explain study of equilibrium diagrams and invariant reactions.
A13)
- Almost all substances have a couple of section in them. Thus engineering substances obtain their unique properties. Macroscopic primary unit of a fabric is referred to as thing.
- It refers to a unbiased chemical species. The components of a machine can be elements, ions or compounds.
- A section may be described as a homogeneous part of a machine that has uniform bodily and chemical traits i.e. it's far a bodily wonderful from other phases, chemically homogeneous and mechanically separable part of a machine.
- A thing can exist in lots of phases. E.g.: Water exists as ice, liquid water, and water vapor. Carbon exists as graphite and diamond.
- When stages are found in a system, it isn't necessary that there be a distinction in each bodily and chemical homes; a disparity in a single or the alternative set of homes is sufficient.
- Answer (liquid or solid) is segment with greater than one aspect; a combination is a cloth with greater than one segment. Solute (minor aspect of in a answer) does not extrade the structural sample of the solvent, and the composition of any answer may be varied. In mixtures, there are one-of-a-kind stages, every with its own atomic arrangement. It is feasible to have a combination of one-of-a-kind solutions
Q14) Explain iron-Iron carbide equilibrium diagram
A14)
- Equilibrium means that adjustments happening in a device due to manner intending in a single course are completely compensated via way of means of adjustments because of the reversal of the manner withinside the device. So it's miles taken into consideration as a dynamic situation of stability among atomic moves wherein the consequent is zero.
- The charges of adjustments of temperature or of composition had been extraordinarily sluggish at some stage in the experimental work, in order that the alloy would “come to relaxation” earlier than a variable consisting of temperature, have been once more changed.
- The situation, therefore, is one in all relaxation in place of change. Equilibrium diagram suggest the following:
1.Temperature at which the stable alloy will begin melting and end melting.
2. Possible segment adjustments so as to arise because the end result of changing the composition or temperature.
- The diagram describes the best situations for 2 or greater stages to exist in equilibrium.
- For example, the water section diagram describes a point (triple point) wherein water can coexist in 3 special stages on the identical time. This takes place at simply above the freezing temperature (0.01°C) and 0.006 atm.
- Uses of Equilibrium Diagram in Metallurgy Development of the brand new alloys on the idea of software requirements.
- Production of those alloys. Development and implementation of suitable warmth remedy methods to enhance the chemical, physical, and mechanical homes of those new alloys.
- Troubleshooting problems that stand up all through using those new alloys, in the long run enhancing product predictability.
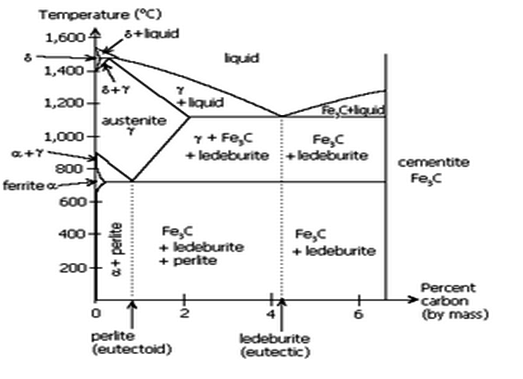
Fig: Iron-Iron carbide equilibrium diagram
- The Iron carbon equilibrium diagram (additionally known as the iron carbon section diagram) is a photograph illustration of the respective microstructure states of the alloy iron – carbon (Fe-C) relying on temperature and carbon content. The iron carbon section diagram is normally used to completely recognize the numerous stages of metal and forged iron.
- Steel and forged iron are each iron and carbon alloys. In addition, each alloys comprise hint factors in small amounts. The graph is pretty complex, however due to the fact we're restricting our research to Fe3C, we can handiest be searching at as much as 6.sixty seven weight percentage carbon.
Q15) What is critical temperature?
A15)
- Critical temperature of metal defines section transition among levels of metal. As the metal is heated above the essential temperature, approximately 1335°F (724°C), it undergoes a section change, recrystallizing as austenite.
- There are varieties of essential temperature: Lower essential temperature (Ac1). The temperature at which austenite begins offevolved to convert from ferrite. Upper essential temperature (Ac3).
- The temperature at which austenite is absolutely converted from ferrite. In the Fe-C system, there may be a eutectoid factor at about 0.8wt% C, 723°C. The section simply above the eutectoid temperature for undeniable carbon steels is called austenite or gamma.
- Steel grading structures don't forget chemical composition, treatment, and mechanical houses to permit fabricators to pick the best product for his or her application.
- Aside from the real percent of carbon and different alloys withinside the material, the microstructure additionally has a enormous affect at the mechanical houses of metal.
- It is vital to recognize the definition of microstructure—and the manner the microstructure of metal may be manipulated the use of warm and bloodless forming and after manufacturing.
- These strategies may be used to expand merchandise with unique mechanical houses. However, manipulating composition and microstructure will bring about a trade-off among one-of-a-kind houses.
- For example, more difficult metal can also additionally become with decreased strength. Microstructure The microstructure of a cloth is the manner wherein the molecules are joined collectively with forces at paintings among the ones molecules.
- Heating and cooling tactics are used to alternate the microstructure from one shape to another, thereby converting the houses of the material. Microstructure isn't observable to the bare eye however may be studied below a microscope. Steel can undertake numerous awesome microstructures—ferrite, pearlite, martensite, cementite, and austenite.
Unit - 1
Introduction to materials
Q1) What is material?
A1)
- Material choice is a totally essential step withinside the method of gadget layout. Selection of cloth relies upon following aspects: Performance Requirements: Material is chosen thinking about the layout constraints and overall performance necessities e.g. Hundreds performing at the member, size & weight constraints, environmental conditions, preferred reliability & sturdiness etc.
- Material Properties: Performance necessities are as compared with homes of substances to choose the nice appropriate cloth. For instance pressure degree expected for any gadget member is as compared with the power of the to be had substances.
- Properties may be Physical (melting point, co-green of thermal expansion, thermal conductivity, particular heat, particular gravity, electric conductivity, magnetic homes etc.), Chemical (corrosion resistance, reactivity with acids, bases, water etc.), Mechanical (hardness, toughness, ductility, malleability etc.) or Manufacturing (castability, weldability, formability, machineability etc.).
- Any of those homes can grow to be essential relying upon the layout necessities and environmental conditions.
- Manufacturing Aspects: Along with the choice of cloth fashion dressmaker additionally has to determine approximately the producing approaches for use to provide it preferred form.
- Therefore further to the producing homes of the cloth, production constraints also are to be taken care of, even as choosing a selected cloth.
- Availability & Cost: Material decided on need to be without difficulty to be had at a suitable fee. In addition to the cloth fee, general fee of fabrication is likewise taken into consideration because the preferred form has to receive to the cloth with nice pleasant and least fee. Availability of a massive quantity of substances with various homes makes the activity of cloth choice very difficult.
Q2) Classify the metal in detail.
A2)
Engineering materials refers to the group of materials that are used in the construction of manmade structures and components. The primary function of an engineering material is to withstand applied loading without breaking and without exhibiting excessive deflection. The major classifications of engineering materials include metals, polymers, ceramics, and composites. The important characteristics of the materials within each of these classes are discussed on this page, and tables of material properties are also provided.
Metals
Metals are the most commonly used class of engineering material. Metal alloys are especially common, and they are formed by combining a metal with one or more other metallic and/or non-metallic materials. The combination usually occurs through a process of melting, mixing, and cooling. The goal of alloying is to improve the properties of the base material in some desirable way.
Metal alloy compositions are described in terms of the percentages of the various elements in the alloy, where the percentages are measured by weight.
Ferrous Alloys
Ferrous alloys have iron as the base element. These alloys and include steels and cast irons. Ferrous alloys are the most common metal alloys in use due to the abundance of iron, ease of production, and high versatility of the material. The biggest disadvantage of many ferrous alloys is low corrosion resistance.
Carbon is an important alloying element in all ferrous alloys. In general, higher levels of carbon increase strength and hardness, and decrease ductility and weldability.
Carbon Steel
Carbon steels are basically just mixtures of iron and carbon. They may contain small amounts of other elements, but carbon is the primary alloying ingredient. The effect of adding carbon is an increase in strength and hardness.
Most carbon steels are plain carbon steels, of which there are several types.
Low-Alloy Steel
Low-alloy steels, also commonly called alloy steels, contain less than about 8% total alloying ingredients. Low-alloy steels are typically stronger than carbon steels and have better corrosion resistance.
Some low-alloy steels are designated as high-strength low-alloy (HSLA) steels. What sets HSLA steels apart from other low-alloy steels is that they are designed to achieve specific mechanical properties rather than to meet a specific chemical composition
Tool Steel
Tool steels are primarily used to make tooling for use in manufacturing, for example cutting tools, drill bits, punches, dies, and chisels. Alloying elements are typically chosen to optimize hardness, wear resistance, and toughness.
Stainless Steel
Stainless steels have good corrosion resistance, mostly due to the addition of chromium as an alloying ingredient. Stainless steels have a chromium composition of at least 11%. Passivation occurs with chromium content at or above 12%, in which case a protective inert film of chromic oxide forms over the material and prevents oxidation. The corrosion resistance of stainless steel is a result of this passivation.
Cast Iron
Cast iron is a ferrous alloy containing high levels of carbon, generally greater than 2%. The carbon present in the cast iron can take the form of graphite or carbide. Cast irons have a low melting temperature which makes them well suited to casting.
Gray Cast Iron
Gray cast iron is the most common type. The carbon is in the form of graphite flakes. Gray cast iron is a brittle material, and its compressive strength is much higher than its tensile strength. The fracture surface of gray cast iron has a gray color, which is how it got its name.
Ductile Cast Iron (Nodular Cast Iron)
The addition of magnesium to gray cast iron improves the ductility of the material. The resulting material is called nodular cast iron because the magnesium causes the graphite flakes to form into spherical nodules. It is also called ductile cast iron. Nodular cast iron has good strength, ductility, and machinability. Common uses include crankshafts, gears, pump bodies, valves, and machine parts.
White Cast Iron
White cast iron has carbon in the form of carbide, which makes the material hard, brittle, and difficult to machine. White cast iron is primarily used for wear-resisting components as well as for the production of malleable cast iron.
Malleable Cast Iron
Malleable cast iron is produced by heat treating white cast iron. The heat treatment improves the ductility of the material while maintaining its high strength.
Aluminum Alloys
Pure aluminum is soft and weak, but it can be alloyed to increase strength. Pure aluminum has good corrosion resistance due to an oxide coating that forms over the material and prevents oxidation. Alloying the aluminum tends to reduce its corrosion resistance.
Aluminum is a widely used material, particularly in the aerospace industry, due to its light weight and corrosion resistance. Despite the fact that aluminum alloys are generally not as strong as steels, they nevertheless have a good strength-to-weight ratio.
Nickel Alloys
Nickel alloys have high temperature and corrosion resistance. Common alloying ingredients include copper, chromium, and iron. Common nickel alloys include Monel, K-Monel, Inconel, and Hastelloy
Copper Alloys
Copper alloys are generally characterized as being electrically conductive, having good corrosion resistance, and being relatively easy to form and cast. While they are a useful engineering material, copper alloys are also very attractive and are commonly used in decorative applications.
Copper alloys primarily consist of brasses and bronzes. Zinc is the major alloying ingredient in brass. Tin is a major alloying element in most bronzes. Bronzes may also contain aluminum, nickel, zinc, silicon, and other elements. The bronzes are typically stronger than the brasses while still maintaining good corrosion resistance.
Titanium Alloys
Titanium alloys are light, strong, and have high corrosion resistance. Their density is much lower than steel, and their strength-to-weight ratio is excellent. For this reason, titanium alloys are used fairly commonly, especially in the aerospace industry. One primary downside of titanium alloys is the high cost.
There are three categories of titanium alloys: alpha alloys, beta alloys, and alpha-beta alloys. Alpha alloys do not respond to heat treatment and are instead strengthened through solid-solution strengthening processes. The beta and alpha-beta alloys can be strengthened by heat treatment, primarily through precipitation hardening.
Q3) What are polymers?
A3)
Polymers
Polymers are materials that consist of molecules formed by long chains of repeating units. They may be natural or synthetic. Many useful engineering materials are polymers, such as plastics, rubbers, fibers, adhesives, and coatings. Polymers are classified as thermoplastic polymers, thermosetting polymers (thermosets), and elastomers.
Thermoplastic Polymers
The classification of thermoplastics and thermosets is based on their response to heat. If heat is applied to a thermoplastic, it will soften and melt. Once it is cooled, it will return to solid form. Thermoplastics do not experience any chemical change through repeated heating and cooling (unless the temperature is high enough to break the molecular bonds). They are therefore very well suited to injection molding.
Thermosetting Polymers
Thermosets are typically heated during initial processing, after which they become permanently hard. Thermosets will not melt upon reheating. If the applied heat becomes extreme however, the thermoset will degrade due to breaking of the molecular bonds. Thermosets typically have greater hardness and strength than thermoplastics. They also typically have better dimensional stability than thermoplastics, meaning that they are better at maintaining their original dimensions when subjected to temperature and moisture changes.
Elastomers
Elastomers are highly elastic polymers with mechanical properties similar to rubber. Elastomers are commonly used for seals, adhesives, hoses, belts, and other flexible parts. The strength and stiffness of rubber can be increased through a process called vulcanization, which involves adding sulfur and subjecting the material to high temperature and pressure. This process causes cross-links to form between the polymer chains.
Ceramics
Ceramics are solid compounds that may consist of metallic or nonmetallic elements. The primary classifications of ceramics include glasses, cements, clay products, refractories, and abrasives.
Ceramics generally have excellent corrosion and wear resistance, high melting temperature, high stiffness, and low electrical and thermal conductivity. Ceramics are also very brittle materials.
Glass
Glasses are common materials and are seen in applications including windows, lenses, and containers. Glasses are amorphous, whereas the other ceramics are mainly crystalline. Primary advantages of glasses include transparency and ease of fabrication. The base element of most glasses is silica, and other components can be added to modify its properties. Common processes used to form glass include:
- Heating until melting, then pouring into molds to cast into useful shapes
- Heating until soft, then rolling
- Heating until soft, then blowing into desired shapes
Cements
Cements are materials that, after mixing with water, form a paste that then hardens. Because of this characteristic, cements can be formed into useful shapes while in paste form before they harden into rigid structures. Plaster of Paris is one common cement. The most common cement is called Portland cement, which is made by mixing clay and limestone and then firing at high temperature. Portland cement is used to form concrete, which is made by mixing it with sand, gravel, and water. It can also be mixed with sand and water to form mortar. Like other ceramics, cements are weak in tension but strong in compression. Cement is very inexpensive to produce, and it is used widely in the construction of buildings, bridges, and other large structures.
Clay Products
Clay is a very common ceramic material. It can be mixed with water, shaped, and then hardened through firing at high temperature. The two primary classifications of clay products include structural clay products and whitewares. Structural clay products see applications including bricks, tiles, and piping. Whitewares see applications including pottery and plumbing fixtures.
Refractories
Refractory ceramics can withstand high temperatures and extreme environments. They can also provide thermal insulation. Brick is the most common refractory ceramic.
Abrasives
Abrasive ceramics are hard materials that are used to cut, grind, and wear away other softer materials. Typical properties of abrasives include high hardness, wear resistance, and temperature resistance. Abrasives can either be bonded to a surface (e.g., grinding wheels and sandpaper), or can be used as loose grains (e.g., sand blasting). Common abrasives include cemented carbide, silicon carbide, tungsten carbide, aluminum oxide, and silica sand. Diamond is also an excellent abrasive, but it is expensive.
Q4) Explain properties and applications of materials.
A4)
Mechanical properties of materials:
- Strength: Ability of material that can resist or withstand mechanical load.
- Ductility: Ability to material to form wires.
- Malleability: Ability of material to form sheets.
- Brittleness: Ability of a material to withstand mechanical load without plastic deformation.
- Hardness: Ability of a material that can offer resistance against mechanical deformation.
- Toughness: Ability of a material that can absorb energy at the time of failure.
- Stiffness: Ability of a material that can resist mechanical deformation under stress
- Resilience: Ability of a material that can absorb energy against failure, without undergoing shape change
Application:
- Atomic Resolution Microscopy: JEOL instrumentation is unequalled for atomic decision imaging. The ultrastable electron column and excessive decision of the ARM200F aberration-corrected TEM push substances studies to new frontiers. The unequalled uncooked facts from the ARM200F reveal never-before-visible imaging decision plus excessive spatial decision for chemical analysis. The ARM200F capabilities an all-new shielded electron column layout that surpasses all different TEM designs today.
- Biomaterials – Borrowing from: Nature Today’s substances technology researchers are main the manner in fabricating newer, more potent substances primarily based totally on those who arise in nature. Nacre, the iridescent, excellentb robust fabric referred to as mother-of-pearl, is sectioned the use of Focused Ion Beam (FIB) and studied the use of SEM for houses which could make a contribution to growing more potent substances at MIT’s Institute for Soldier Nanotechnologies. A flexible JEOL analytical, area emission TEM, the JEM-3200FS, is utilized in multidisciplinary research at Indiana University to increase self-assembled molecular layers and nanoparticles that mimic the self-meeting of viruses.
- Pioneering Nanotechnology :JEOL TEMs and SEMs have lengthy been used to advantage superior studies in addition to failure analysis. In the 1980s, a JEOL excessive decision TEM demonstrated the shape of C60 and helped result in early discoveries in carbon nanotubes. Nobel prize winners, recipients of fundamental studies grants, and world-famend scientists have used JEOL gadgets of their superior studies.
- Structural Imaging and Analysis: Microstructural records and surface/bulk chemical analyses are simply received from JEOL SEMs and Microprobes with ultra-modern results. Elemental mapping and characterization of high-quality systems are recurring for JEOL’s excessive overall performance FEG-SEMs, which includes the ultrahigh decision analytical area emission JSM-7600F. Coatings, adhesives, layers, composites – all is discovered through the electricity of the SEM and Microprobe.
- Nanofabrication: Versatile SEM/FIB tools, just like the JEOL MultiBeam, permit simultaneous viewing, analysis, and micro milling functions, and serial reducing and sampling (S3) for monitoring, reducing, fabrication and reconstruction of specimens in 3D. JEOL’s information in e-beam lithography expands the functionality of the Field Emission SEM to permit for direct write patterning and gas-assisted e-beam lithography. In the 1960s, direct write e-beam turned into used for writing IC circuits on small wafers for a committed application. Now it's miles utilized in a myriad of programs from photonics and DNA filters to nano-fluidics, nano-hole patterns, and unmarried electron transistors.
Q5) Explain crystalline nature of metals.
A5)
- Metal elements (besides Cs, Ga, and Hg) are crystalline solids at room temperature. Like ionic solids, metals and alloys have a totally sturdy tendency to crystallize, whether or not they may be made with the aid of using thermal processing or with the aid of using different strategies which include answer discount or electroplating.
- Metals crystallize without problems and it's far hard to shape a glassy metallic despite very speedy cooling. Molten metals have low viscosity, and the identical (basically spherical) atoms can % right into a crystal very easily. Glassy metals may be made, however, with the aid of using hastily cooling alloys, especially if the constituent atoms have one of a kind sizes.
- The one of a kind atoms cannot % in a easy unit cell, on occasion making crystallization sluggish sufficient to shape a glass. Most metals and alloys crystallize in certainly considered one among 3 very not unusualplace systems: body-focused cubic (bcc), hexagonal near packed (hcp), or cubic near packed (ccp, additionally known as face focused cubic, fcc).
- In all 3 systems the coordination quantity of the metallic atoms (i.e., the quantity of equidistant nearest associates) is alternatively high: eight for bcc, and 12 for hcp and ccp.
- We can evaluation this with the low coordination numbers (i.e., low valences - like 2 for O, three for N, or four for C) observed in nonmetals. In the bcc structure, the closest associates are on the corners of a dice surrounding the metallic atom withinside the center. In the hcp and ccp systems, the atoms % like stacked cannonballs or billiard balls, in layers with a six-coordinate arrangement.
- Each atom additionally has six extra nearest associates from layers above and below.
- The stacking series is ABCABC... With inside the ccp lattice and ABAB... In hcp. In each cases, it may be proven that the spheres fill 74% of the extent of the lattice. This is the best extent fraction that may be packed with a lattice of identical spheres.
- Atoms in steel crystals will be inclined to % in dense arrangments that fill area successfully. The easy rectangular packing (above) upon which the easy cubic shape is primarily based totally is inefficient and as a consequence uncommon amongst steel crystal systems. Body- or face-focused systems fill area greater successfully and greater common.
Q6) Write a note on specially microscopic and macroscopic examinations of metals.
A6)
- Metallography is the have a look at of the bodily shape and additives of metals, through the use of microscopy.
- Ceramic and polymeric substances can also be organized the use of metallographic techniques, therefore the phrases ceramography, plastography and, collectively, materialography.
- The floor of a metallographic specimen is ready through diverse techniques of grinding, sharpening, and etching. After coaching, it's far frequently analyzed the use of optical or electron microscopy.
- Using simplest metallographic techniques, a professional technician can perceive alloys and expect fabric properties. Mechanical coaching is the maximum not unusualplace coaching approach. Successively finer abrasive debris are used to put off fabric from the pattern floor till the favored floor exceptional is achieved. Many unique machines are to be had for doing this grinding and sharpening, which might be capable of meet unique needs for exceptional, capacity, and reproducibility.
- A systematic coaching approach is the perfect manner to obtain the authentic shape. Sample coaching should consequently pursue policies which might be appropriate for maximum substances. Different substances with comparable properties (hardness and ductility) will reply alike and accordingly require the equal consumables in the course of coaching.
- Metallographic specimens are typically "mounted" the use of a warm compression thermosetting resin. In the past, phenolic thermosetting resins were used, however current epoxy is turning into extra famous due to the fact decreased shrinkage in the course of curing outcomes in a higher mount with advanced part retention.
- A ordinary mounting cycle will compress the specimen and mounting media to 4,000 psi (28 MPa) and warmth to a temperature of 350 °F (177 °C). When specimens are very touchy to temperature, "bloodless mounts" can be made with a two-element epoxy resin.
- Mounting a specimen offers a safe, standardized, and ergonomic manner through which to keep a pattern in the course of the grinding and sharpening operations. A macro etched copper disc After mounting, the specimen is moist floor to show the floor of the metallic.
- The specimen is successively floor with finer and finer abrasive media. Silicon carbide abrasive paper became the primary approach of grinding and remains used today. Many metallographers, however, choose to use a diamond grit suspension that's dosed onto a reusable material pad during the sharpening process.
- Diamond grit in suspension may begin at nine micrometres and end at one micrometre. Generally, sharpening with diamond suspension offers finer outcomes than the use of silicon carbide papers (SiC papers), mainly with revealing porosity, which silicon carbide paper sometimes "smear" over. After grinding the specimen, sharpening is performed.
- Typically, a specimen is polished with a slurry of alumina, silica, or diamond on a napless fabric to supply a scratch-unfastened replicate end, unfastened from smear, drag, or pull-outs and with minimum deformation last from the coaching process.
- After sharpening, sure microstructural parts may be visible with the microscope, e.g., inclusions and nitrides. If the crystal shape is non-cubic (e.g., a metallic with a hexagonal-closed packed crystal shape, which include Ti or Zr) the microstructure may be discovered with out etching the use of crossed polarized mild (mild microscopy).
- Otherwise, the microstructural parts of the specimen are discovered through the use of a appropriate chemical or electrolytic etchant.
Q7) Write a short note on ferrous alloy.
A7)
Ferrous alloys:
Those in which iron is the primary component are produced in greater quantities than any other metal. They're particularly useful as engineering building materials. Three variables account for their extensive use: (1) iron-containing compounds are abundant in the earth's crust; (2) metallic iron and steel alloys can be made with relatively low-cost extraction, refining, alloying, and fabrication techniques; and (3) ferrous alloys are extremely versatile, as they can be tailored to have a wide range of mechanical and physical properties.
The main disadvantage of many ferrous alloys is their corrosion resistance. This section covers the compositions, microstructures, and properties of a variety of steels and cast irons.
Q8) What is steel?
A8)
Steels:
Steels are iron–carbon alloys that may contain significant amounts of additional alloying elements; there are thousands of distinct alloys with various compositions and heat treatments. The mechanical properties are affected by the carbon content, which is typically less than 1.0 wt%. Some of the more prevalent steels are divided into low-, medium-, and high-carbon categories based on carbon content. Within each group, subclasses exist based on the concentration of various alloying elements. Other than carbon and a small amount of manganese, plain carbon steels contain only residual impurities. More alloying elements are purposely introduced in specified concentrations to alloy steels.
1) Low carbon steel:
Low-carbon steels account for the majority of the numerous types of steel manufactured. These typically contain less than 0.25 wt% C and are insensitive to heat treatments designed to create martensite; cold work is used to reinforce them. The elements of microstructures are ferrite and pearlite. As a result, these alloys are relatively soft and weak but have exceptional ductility and toughness; they are also machinable, weldable, and the least expensive to create of all steels.
Automobile body components, structural shapes (I-beams, channel and angle iron), and sheets used in pipelines, buildings, bridges, and tin cans are only a few examples. They have a yield strength of 275 MPa (40,000 psi), tensile strengths of 415 to 550 MPa (60,000 to 80,000 psi), and 25 percent EL ductility. The high-strength, low-alloy (HSLA) steels are another type of low-carbon alloy. They have higher strengths than ordinary low-carbon steels because they incorporate various alloying elements such as copper, vanadium, nickel, and molybdenum in combined concentrations as high as 10% by weight.
Heat treatment can increase tensile strength to above 480 MPa (70,000 psi); they are also ductile, formable, and machinable. In normal environments, HSLA steels are more corrosion resistant than ordinary carbon steels, which they have largely supplanted in many applications where structural strength is required (for example, bridges, towers, high-rise building support columns, and pressure vessels).
2) Medium-Carbon Steels
Carbon contents in medium-carbon steels range from 0.25 to 0.60 wt percent. To improve their mechanical properties, these alloys can be heat-treated by austenitizing, quenching, and tempering. They are most used in tempered form, with tempered martensite microstructures. Plain medium-carbon steels have low hardenability and can only be heat-treated satisfactorily in very thin sections and at extremely high quenching rates.
Additions of chromium, nickel, and molybdenum improve the alloys' heat-treatability, resulting in a wide range of strength–ductility combinations. These heat-treated alloys are stronger than low-carbon steels, but ductility and toughness are sacrificed. Railway wheels and tracks, gears, crankshafts, and other machine elements, as well as high-strength structural components, all require a blend of strength, wear resistance, and toughness.
3) High-Carbon Steels
High-carbon steels, with carbon levels typically ranging from 0.60 to 1.4 wt%, are the hardest, strongest, and least ductile of the carbon steels. They are virtually always hardened and tempered, which makes them particularly wear resistant and capable of keeping a sharp cutting edge. High-carbon alloys with chromium, vanadium, tungsten, and molybdenum are used in tool and die steels. These alloying elements mix with carbon to generate carbide compounds that are extremely hard and wear resistant.
These steels are used in knives, razors, hacksaw blades, springs, and high-strength wire, as well as cutting tools and dies for moulding and shaping materials.
4) Stainless Steels
In a range of conditions, including the ambient atmosphere, stainless steels are highly resistant to corrosion (rusting). The most common alloying element is chromium, which must have a concentration of at least 11 weight percent Cr. Nickel and molybdenum additives can also improve corrosion resistance. On the basis of the major phase composition of the microstructure, stainless steels are classified as martensitic, ferritic, or austenitic. Stainless steels are extremely adaptable in their use due to their wide range of mechanical qualities and good corrosion resistance. Heat-treating martensitic stainless steels to make martensite the predominant microconstituent is possible. The iron–iron carbide phase diagram changes dramatically when alloying metals are added in considerable amounts.
Q9) What is cast iron?
A9)
Cast Iron
Cast irons are a type of ferrous alloy with a carbon concentration greater than 2.14 weight percent; however, most cast irons possess between 3.0 and 4.5 weight percent C, as well as other alloying elements. A closer look at the iron–iron carbide phase diagram reveals that alloys in this composition range become entirely liquid at temperatures between 1150 and 1300C (2100 and 2350F), which is significantly lower than steel temperatures. As a result, they are easily melted and castable. Furthermore, some cast irons are extremely fragile, and casting is the most practical method of manufacturing.
Gray iron
Gray cast irons have carbon and silicon concentrations of 2.5 to 4.0 wt percent and 1.0 to 3.0 wt percent, respectively. The graphite in most of these cast irons is in the form of flakes (like corn flakes), which are usually surrounded by an x-ferrite or pearlite matrix; this is the microstructure of a typical grey iron. A shattered surface takes on a grey look as a result of the graphite flakes, hence the name.
Solid solutions
When homogeneous mixtures of two or more kinds of atoms (of metal) occur in the solid state, they are known as the solid solutions.
The more abundant atomic form is referred as solvent and less abundant atomic form is referred as solute.
For example: - In sterling silver (92.5% of silver of remaining is copper) is a solid solution of silver and copper where silver is solvent and copper is solute.
Types of solid solutions: -
Solid solutions are generally of two types
1) Substitutional solid solutions.
2) Interstitial solid solutions
Substitutional solid solution:
As suggested by the name substitutional means 'replacement’. Here in this case when the atom of the parent metal or we can say atom of the solvent replaced by the atom of the solute metal then the solid solution is known as substitutional solid solution.
Hume Rothery rules for formation of substitutional solid solutions:
Hume Rothery rules defines us the various conditions under which an element court dissolve in a metal forming a solid solution.
These are as follows:
a) Crystal structure factor
b) Relative size factor
c) Chemical affinity sector
d) Relative valence factor
Crystal structure factor: - The crystal structure of solute and solvent must be similar. Example: - either crystal structure should be FCC, or BCC or HCP.
Relative size factor: - The atomic radius of the solute and solvent atoms must differ by not more than 15%. If it’s beyond 15% then it restricts solid solubility.
Chemical affinity factor: - When two metals have lesser chemical affinity then it forms solid solution. If there will be greater chemical affinity then it can cause compound formation. In others we can see that the solute and solvent should have similar electronegativity.
Relative valence factor: - The complete solubility occurs when solute and solvent must have same valency. A metal with lower valency is more likely to dissolve in metal of higher valency.
(2) Interstitial solid solutions: -
In interstitial solid solutions the solute atom does not displace a solvent atom but rather it enters one of the holes or interstices between the solvent atoms.
Q10) Explain types and their formations of steel.
A10)
- There are kinds of strong solutions: 1. Substitutional Solid Solution 2. Interstitial Solid Solutions. Solute is the minor detail this is delivered to the solvent, and solvent is the important detail of answer.
- When a specific crystal shape of the solvent is maintained at some point of alloying the alloy is referred to as a strong answer. The quantity of solute that can be dissolved through the solvent is commonly a feature of temperature (with strain constant) and normally growth with temperature. There are 3 feasible situation for answer i.e., unsaturated, saturated and supersaturated.
Type 1. Substitutional Solid Solution:
- If the scale of the solute atom is just like that of the solvent atom, the solute atoms can update solvent atoms to shape a substitutional stable solution. Example: Brass, wherein zinc (solute atom) is added into the lattice of copper (Solvent).
- Two situations are typically required to from entire substitutional stable solution:
(i) Two factors have to have comparable crystal structure.
(ii) The distinction of their atomic radii need to be much less than 15%. Example: In the Aluminium nickel alloy system, each steel are FCC. The relative length component is 14%. However, nickel is decrease in valence then Al and as in step with relative valence component stable nickel dissolve 5% Al however better valence Al, dissolve handiest 0.04 % Ni.
Type 2. Interstitial Solid Solutions:
- If the scale of the solute atom is plenty smaller than that of the solvent atom the solute atom can occupy an interstitial role forming interstitial stable solution. Since the distance of the lattice shape are confined in size, most effective atoms with atomic radii much less than 1 angstrom are usually shape interstitial stable solution.
- These are hydrogen (0.46), boron (0.97), Carbon (0.77), Nitrogen (0.71) and Oxygen (0.60). More solute atoms can be dissolved interstitially till the answer emerge as saturated at that temperature. Interstitial stable answers usually have constrained solubility and usually are of a touch importance. Carbon in iron is a exquisite exception and paperwork the premise for hardening steel. Carbon dissolves in iron interstitially.
- The most solubility of Carbon in iron (F.C.C.) is 2% at 1150°F at the same time as most solubility of carbon in α iron (B.C.C.) is most effective 0.025% at 727°C. Two important situations for forming ISS are: (i) The solvent atom ought to have a couple of valence. (ii) The atomic radius of solute atom ought to be much less than 59% of the atomic radii of solvent atom. Example: Steel, wherein carbon atoms are found in interstitial positions among iron atoms with most percent of 2. Atomic radius of carbon is 0.071 nm that is much less than 59% of 0.one hundred twenty five nm radius of iron atom.
Formation of solid solution:
- Stable answer, combination of crystalline solids that coexist as a brand new crystalline stable, or crystal lattice.
- The blending may be done through combining the 2 solids after they were melted into drinks at excessive temperatures after which cooling the end result to shape the brand new stable or through depositing vapours of the beginning substances onto substrates to shape skinny films. As with drinks, solids have one of a kind levels of mutual solubility, relying on their chemical residences and crystalline structure, which decide how their atoms suit collectively withinside the blended crystal lattice.
- The blended lattice can be substitutional, wherein the atoms of 1 beginning crystal update the ones of the other, or interstitial, wherein the atoms occupy positions commonly vacant withinside the lattice.
- The materials can be soluble over a partial or maybe whole variety of relative concentrations, generating a crystal whose residences range constantly over the variety.
- This presents a manner to tailor the residences of the stable answer for unique applications. Many stable answers seem in nature withinside the shape of minerals made below situations of warmth and pressure.
- One instance is the olivine mineral group, specially the forsterite-fayalite series, whose individuals range from forsterite (Mg2SiO4) to fayalite (Fe2SiO4).
- The compounds have same crystal systems and shape a substitutional stable answer that may variety from a hundred percentage magnesium (Mg) to a hundred percentage iron (Fe), along with all proportions in between, with bodily residences that change easily from the ones of forsterite to the ones of fayalite.
Q11) Explain modified Gibbs’s phase rule in detail.
A11)
- Gibbs segment rule states that if the equilibrium in a heterogeneous machine isn't stricken by gravity or through electric and magnetic forces, the wide variety of diploma of freedom is given through the equation
F=C-P+2
- Wherein C is the wide variety of chemical components P is the wide variety of levels Basically, it describes the mathematical dating for figuring out the steadiness of levels gift withinside the fabric at equilibrium condition. In the subsequent section, allow us to study the segment rule derivation.
- The Gibb’s section rule on the idea of the thermodynamic rule may be derived as follows: First, allow us to do not forget a heterogeneous device including Pn range of stages and Cn range of additives in equilibrium. Let us anticipate that the passage of a element from one section to any other doesn’t contain any chemical reaction.
- When the device is in equilibrium, it is able to be defined via way of means of the subsequent parameters: Temperature Pressure The composition of every section a. The general range of variables required to specify the nation of the device is: Pressure: identical for all stages Temperature: identical for all stages Concentration The impartial awareness variables for one section with admire to the C additives is C – 1. Therefore, the impartial awareness variables for P stages with admire to C additives is P (C – 1).
Total number of variables = P (C – 1) + 2 (1)
The total number of equilibria:
- The various phases present in the system can only remain in equilibrium when the chemical potential (µ) of each of the component is the same in all phases, i.e.
µ1, P1 = | µ1, P2 = | µ1, P3 = | … | = | µ1, P |
µ2, P1 = | µ2, P2 = | µ2, P3 = | … | = | µ2, P |
: | : | : |
|
| : |
: | : | : |
|
| : |
: | : | : |
|
| : |
µC, P1 = | µC, P2 = | µC, P3 = | … | = | µC, P |
The number of equilibria for each P phases for each component is P – 1.
For C components, the number of equilibria for P phases is P ( C – 1).
Hence, the total number of equilibria involved is E = C (P – 1). (2)
Equating eq (1) and (2), we get
F=[P(C−1)+2−C]−[C(P−1)]F=[P(C−1)+2−C]−[C(P−1)] F=[CP−P+2−CP+C]F=[CP−P+2−CP+C] F=C−P+2F=C−P+2
The obtained formula is the Gibbs phase rule. Stay tuned to BYJU’S to learn more physics derivations.
Q12) What is lever rule for phase mixtures and their application in system?
A12)
- If an alloy includes a couple of section, the quantity of every section gift may be located through making use of the lever rule to the section diagram. The lever rule may be defined through thinking about a easy stability.
- The composition of the alloy is represented through the fulcrum, and the compositions of the 2 levels through the ends of a bar. The proportions of the levels gift are decided through the weights had to stability the system.

Fig: Lever rule
So,
Fraction of phase 1 (C2 - C) / (C2 - C1)
And,
Fraction of phase 2 (C - C1) / (C2 - C1).
- Point 1 At factor 1 the alloy is absolutely liquid, with a composition C. Let C = sixty five weight% B.
- Point 2 At factor 2 the alloy has cooled as a ways because the liquidus, and strong section β begins offevolved to form. Phase β first bureaucracy with a composition of ninety six weight% B.
- The inexperienced dashed line underneath is an instance of a tie-line. A tie-line is a horizontal (i.e., constant-temperature) line via the selected factor, which intersects the section boundary strains on both side.
Q13) Explain study of equilibrium diagrams and invariant reactions.
A13)
- Almost all substances have a couple of section in them. Thus engineering substances obtain their unique properties. Macroscopic primary unit of a fabric is referred to as thing.
- It refers to a unbiased chemical species. The components of a machine can be elements, ions or compounds.
- A section may be described as a homogeneous part of a machine that has uniform bodily and chemical traits i.e. it's far a bodily wonderful from other phases, chemically homogeneous and mechanically separable part of a machine.
- A thing can exist in lots of phases. E.g.: Water exists as ice, liquid water, and water vapor. Carbon exists as graphite and diamond.
- When stages are found in a system, it isn't necessary that there be a distinction in each bodily and chemical homes; a disparity in a single or the alternative set of homes is sufficient.
- Answer (liquid or solid) is segment with greater than one aspect; a combination is a cloth with greater than one segment. Solute (minor aspect of in a answer) does not extrade the structural sample of the solvent, and the composition of any answer may be varied. In mixtures, there are one-of-a-kind stages, every with its own atomic arrangement. It is feasible to have a combination of one-of-a-kind solutions
Q14) Explain iron-Iron carbide equilibrium diagram
A14)
- Equilibrium means that adjustments happening in a device due to manner intending in a single course are completely compensated via way of means of adjustments because of the reversal of the manner withinside the device. So it's miles taken into consideration as a dynamic situation of stability among atomic moves wherein the consequent is zero.
- The charges of adjustments of temperature or of composition had been extraordinarily sluggish at some stage in the experimental work, in order that the alloy would “come to relaxation” earlier than a variable consisting of temperature, have been once more changed.
- The situation, therefore, is one in all relaxation in place of change. Equilibrium diagram suggest the following:
1.Temperature at which the stable alloy will begin melting and end melting.
2. Possible segment adjustments so as to arise because the end result of changing the composition or temperature.
- The diagram describes the best situations for 2 or greater stages to exist in equilibrium.
- For example, the water section diagram describes a point (triple point) wherein water can coexist in 3 special stages on the identical time. This takes place at simply above the freezing temperature (0.01°C) and 0.006 atm.
- Uses of Equilibrium Diagram in Metallurgy Development of the brand new alloys on the idea of software requirements.
- Production of those alloys. Development and implementation of suitable warmth remedy methods to enhance the chemical, physical, and mechanical homes of those new alloys.
- Troubleshooting problems that stand up all through using those new alloys, in the long run enhancing product predictability.
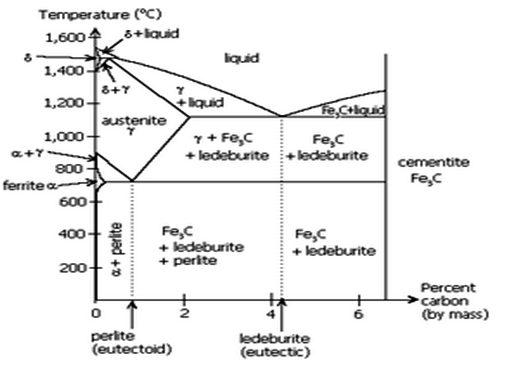
Fig: Iron-Iron carbide equilibrium diagram
- The Iron carbon equilibrium diagram (additionally known as the iron carbon section diagram) is a photograph illustration of the respective microstructure states of the alloy iron – carbon (Fe-C) relying on temperature and carbon content. The iron carbon section diagram is normally used to completely recognize the numerous stages of metal and forged iron.
- Steel and forged iron are each iron and carbon alloys. In addition, each alloys comprise hint factors in small amounts. The graph is pretty complex, however due to the fact we're restricting our research to Fe3C, we can handiest be searching at as much as 6.sixty seven weight percentage carbon.
Q15) What is critical temperature?
A15)
- Critical temperature of metal defines section transition among levels of metal. As the metal is heated above the essential temperature, approximately 1335°F (724°C), it undergoes a section change, recrystallizing as austenite.
- There are varieties of essential temperature: Lower essential temperature (Ac1). The temperature at which austenite begins offevolved to convert from ferrite. Upper essential temperature (Ac3).
- The temperature at which austenite is absolutely converted from ferrite. In the Fe-C system, there may be a eutectoid factor at about 0.8wt% C, 723°C. The section simply above the eutectoid temperature for undeniable carbon steels is called austenite or gamma.
- Steel grading structures don't forget chemical composition, treatment, and mechanical houses to permit fabricators to pick the best product for his or her application.
- Aside from the real percent of carbon and different alloys withinside the material, the microstructure additionally has a enormous affect at the mechanical houses of metal.
- It is vital to recognize the definition of microstructure—and the manner the microstructure of metal may be manipulated the use of warm and bloodless forming and after manufacturing.
- These strategies may be used to expand merchandise with unique mechanical houses. However, manipulating composition and microstructure will bring about a trade-off among one-of-a-kind houses.
- For example, more difficult metal can also additionally become with decreased strength. Microstructure The microstructure of a cloth is the manner wherein the molecules are joined collectively with forces at paintings among the ones molecules.
- Heating and cooling tactics are used to alternate the microstructure from one shape to another, thereby converting the houses of the material. Microstructure isn't observable to the bare eye however may be studied below a microscope. Steel can undertake numerous awesome microstructures—ferrite, pearlite, martensite, cementite, and austenite.