Unit - 3
Heat treatment and its importance
Q1) Explain heat treatment and its importance in detail.
A1)
- All heating processes are almost similar because all the processes involve heating and cooling. The things which makes difference in it is cooling temperature or say cooling rate, heating temperature and also quenching medium to achieve desired properties.
- The heat treatment of ferrous metals (metals with iron) usually consist of annealing, normalizing and tempering.
- Most nonferrous metals can be annealed but never tempered, normalized for case hardened.
Heat treatment process: -
1) Hardening- Hardness is usually followed by tempering to minimize brittleness. Steel hardening is carried out by heating to temperature above critical temperature.
- Done quenching in water, oil.
- Final microstructure is martensite.
2) Tempering: - After hardening tempering is done.as we get hard and brittle structure while hardening so after secondary heating of martensite is done to get desired ductility, hardness and strength.
In tempering ductility increases with slight loss in hardness and steel.
3) Annealing: -
- Annealing involves heating the material to predetermined temperature and hold the material at the temperature and cool at room temperature i.e. slowly. It helps to relieve internal stresses, improve ductility and toughness, refrain grain size, enhance machinability.
Different types of annealing processes: -
a) Full annealing- In full annealing steel is heated above upper critical temperature i.e. 723°C. Heating will be done nearly at 800°C i.e. 50-70°C above the critical temperature depending upon the carbon percentage.
After heating we will cool at very slowly then it will become ductile in case of hypoeutectoid Steel and in hypereutectoid we will get cementite + pearlite.
After full annealing machinability increases and becomes more ductile.
b) Process annealing: - Done at 550-650°C done below lower critical temperature.
In hypoeutectoid steel (where carbon percentage is less than 0.8%) and hypereutectoid steel (carbon percentage more than 0.8%) in both cases heating done below lower critical temperature. It is done to improve refrain grain size and improves ductility.
c) Spherodise annealing: - It is done on the metals which are very hard to machine. It is done at lower critical temperature i.e. 723-770°C. Here we heat steel and then do slowly cooling by which carbide forms sphere structure. It is mainly done to enhance machinability which are very hard to do machining.
d) Isothermal annealing: - It is very much similar to full annealing process. But the major difference in it is that in case of full annealing hypoeutectoid and hypereutectoid can be done but isothermal annealing is done only add hypoeutectoid Steel.
Heating is done above 723 degree Celsius. It is done to reduce annealing time and to produce homogeneous structure in the material and also to improve machinability.
e) Stress relieving annealing: - As name suggest, this process is done to relieve internal stresses. No microstructural changes occurred during the process.
It is done at lower critical temperature followed by a uniform cooling.
4) Normalizing: -
Heated beyond upper critical temperature and cooled in still air. Soap rate of cooling gets increased. Get harder and structure than annealing.
5) Austempering: - Heating above critical temperature to make it austenitic. Then it is quenched at critical rate in to Salt but held at Bainite range (200-420°C). Austenite is completely transformed into Bainite then cooled at room temperature.
6) Martempering: - Heated above critical range to make it into Austenite and then quenched into salt bath and maintaining temperature for long time during transformation so that there is least internal stresses and distortion. They are cooled in air to room temperature.
7) Sub-zero Treatment: -Sub-zero treatment are treatments in which components are cooled below room temperature. There can be many reasons, but the main reason is to remove retained austenite from quenched components, to increase the wear resistance or to stabilise the component. It is carried out in order to complete the transformation of retained austenite to martensite after hardening and before tempering. It is usually applied to high carbon, high alloy steel but is more widely applied by aerospace companies.
Q2) Explain hardenability.
A2)
Hardenability:
- The hardenability of a metal alloy is the depth to which a material is hardened after putting it through a heat treatment process.
- It should not be confused hardness which is a measure of samples resistance to indentation or scratching.
- It is an important property of welding since it is inversely proportional to weldability that is the ease of welding a material. Hardenability is a term that is used to describe a given steels ability to harden it doesn't mean what hardness can be achieved.
- Hardness is dependent mostly on a ferrous material’s carbon content. The major factor which affects hardenability and the rate of austenite transformation are carbon content, grain size and alloying element.
Q3) Explain annealing
A3)
- When a material is plastically strained the yield, stress is increased. In many of the manufacturing techniques listed earlier, the material is plastically deformed in order to fabricate the desired shape.Aalthough this may significantly increase the yield strength of the material, it also makes it more brittle.
- This change is a result of the dislocation density, or imperfections in the lattice, increasing as a result of the deformation.
- If we take a work-hardened material and subject it to a sufficiently high temperature for a specified time, we can reduce the dislocation density and the yield stress will return to its initial value.
- This is known as annealing the material, which is a form of heat treatment. Due to other aspects, such as recrystallization, subjecting the work-hardened material to a high temperature for too long
Q4) What is normalizing
A4)
- Steels that have undergo plastic deformation consist of pearlites which are irregularly shaped and relatively large, but varying in size. Normalizing is a heat treatment used on steel so as to refine its crystal structure and produces a more uniform and desired grain size distribution. Fine grained pearlites are tougher than coarse grained ones.
- Normalization eliminates internal stresses, strains and improves the mechanical properties of the steel, such as improving its toughness and machinability. A better ductility can also be obtained without compromising the hardness and strength.
- Normalizing is accomplished by heating the steel to a temperature above the transformation range and into the range of complete austenite. This is dependent on the composition of the steel as indicated by the iron-carbon diagram shown below.
- The usual normalizing temperature ranges from 815°C to 980°C (1500°F to 1800°F), depending on the steel involved.
- After sufficient time is given for complete transformation to austenite, i.e., austenitizing of the steel, the alloy is air-cooled to a temperature substantially below the transformation range. The air-cooling avoids excessive proeutectoid segregation. The cooling rate is usually in the range of 500 to 1000 °C/h (900 to 1800 °F/h).
- The final microstructure consists of fine pearlite and an absence of massive proeutectoid ferrite. Normalizing is commonly specified for plates of pressure vessel quality hence to ASTM standards above 1 ½ inch in thickness
Q5) What is hardening?
A5)
- The development of extremely small uniformly dispersed particles of a second phase within the original phase matrix can improve the strength and hardness of particular metal alloys; this must be accomplished by phase transformations induced by proper heat treatments.
- Precipitation hardening is the name given to the process since the new phase's microscopic particles are called precipitates. The term "age hardening" is also used to describe this operation because the alloy's strength increases over time.
- Aluminum–copper, copper–beryllium, copper–tin, and magnesium–aluminum alloys are examples of precipitation hardenable alloys; some ferrous alloys are also precipitation hardenable.
- Even though the heat treatment procedures are similar, precipitation hardening and the treatment of steel to generate tempered martensite are two distinct phenomena that should not be conflated. The main distinction is in the processes used to accomplish hardening and strengthening. As precipitation hardening is explained, they should become clear.
Q6) What is quench cracks?
A6)
- Quench cracks end result from stresses produced at some point of the transition from Austenite to Martensite, which includes an growth in quantity. The martensitic transformation begins offevolved on the outermost surfaces of the element being quenched.
- As the transformation is going deeper into the softer austenite closer to middle of mass, its alternate in quantity is limited via way of means of the martensite already created withinside the outer volumes of the element adjoining to the floor.
- This creates inner stresses which region the floor into tension. When sufficient martensite has shaped to create inner strain extra than the last strength (tensile strength) of the as quenched martensite on the floor, a crack results. As-quenched Martensite is difficult and brittle- it has genuinely no ductility.
Q7) Explain hardenability test
A7)
- Hardenability is a measure that relates the effect of alloy composition on a steel alloy's ability to transition to martensite for a specific quenching procedure. There is a unique relationship between mechanical qualities and cooling rate for each distinct steel alloy.
- The ability of an alloy to be hardened by the development of martensite as a result of a specific heat treatment is referred to as hardenability.
- Hardenability is a qualitative measure of the rate at which hardness drops down with distance into the interior of a specimen as a result of decreasing martensite content, rather than "hardness," which is the resistance to indentation. A steel alloy with a high hardenability hardens, or develops martensite, not only on the surface but also throughout the inside to a great extent.
Q8) Explain TTT diagram and its construction and related Heat Treatment Processes.
A8)
TTT diagram:
- T-T-T diagram is likewise known as isothermal transformation diagram [Temperature-Time –Transformation]. It is a plot of temperature as opposed to the logarithm of time for a metallic alloy of specific composition.
- It is used to decide while ameliorations start and cease for an isothermal [constant thermal] warmth remedy of a formerly austenitized alloy.
- Time-Temperature-Transformation (TTT) diagram or S-curve refers to simplest one metallic of a selected composition at a time, which applies to all carbon steels. This diagram is likewise referred to as as C-curve isothermal (decomposition of austenite) diagram and Bain’s curve.
- The impact of time-temperature at the microstructure adjustments of metallic may be proven through the TTT diagram.
- These diagrams are significantly used withinside the evaluation of the decomposition of austenite in warmth-treatable steels. We have visible that the iron-carbon section diagram does now no longer display time as a variable and subsequently the outcomes of various cooling prices at the systems of steels aren't revealed. Moreover, equilibrium situations aren't maintained in warmth treatment.
- Although, the iron-carbon equilibrium diagram well-knownshows at the stages and corresponding microstructures below equilibrium situations however numerous beneficial residences of the steels may be acquired below non-equilibrium situations, e.g. Variable prices of cooling as produced at some point of quenching and higher transformation of austenite into pearlite and martensite.
Construction:
- For every metallic composition, special IT diagram is acquired. Fig suggests the TTT diagram of eutectoid metallic (i.e. metallic containing 0.8% C). Austenite is strong above eutectoid temperature 727 °C. When metallic is cooled to a temperature beneathneath this eutectoid temperature, austenite is converted into its transformation product.
- TTT diagram relates the transformation of austenite to time and temperature situations. Thus, the TTT diagram suggests transformation merchandise in keeping with temperature and additionally the time required for entire transformation. Curve 1 is transformation start curve even as curve 2 is the transformation quit curve.
- The location to the left of curve 1 corresponds to austenite (A’). The location to the proper of curve 2 represents the entire transformation of austenite (F+C). The c programming language among those curves suggests partial decomposition of austenite into ferrite and Cementite (A’+F+C).
Heat treatment process:
- Heat treating (or warmness remedy) is a collection of industrial, thermal and metalworking procedures used to regulate the physical, and every so often chemical, homes of a material. The maximum not unusualplace utility is metallurgical. Heat remedies also are used withinside the manufacture of many different materials, inclusive of glass. Heat remedy entails using heating or chilling, typically to excessive temperatures, to obtain the preferred end result inclusive of hardening or softening of a material. Heat remedy strategies consist of annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching.
- Although the time period warmness remedy applies handiest to procedures in which the heating and cooling are performed for the unique motive of changing homes intentionally, heating and cooling frequently arise by the way throughout different production procedures inclusive of warm forming or welding.
- Metallic substances encompass a microstructure of small crystals referred to as "grains" or crystallites. The nature of the grains (i.e. grain length and composition) is one of the only elements that may decide the general mechanical conduct of the metallic. Heat remedy affords an green manner to control the houses of the metallic with the aid of using controlling the fee of diffusion and the fee of cooling in the microstructure. Heat treating is regularly used to adjust the mechanical houses of a metal alloy, manipulating houses along with the hardness, strength, toughness, ductility, and elasticity.
- There are mechanisms which can alternate an alloy's houses at some stage in warmness remedy: the formation of martensite reasons the crystals to deform intrinsically, and the diffusion mechanism reasons adjustments withinside the homogeneity of the alloy. The crystal shape includes atoms which might be grouped in a completely particular arrangement, referred to as a lattice. In maximum factors, this order will rearrange itself, relying on situations like temperature and pressure.
- This rearrangement referred to as allotropy or polymorphism, may also arise numerous times, at many unique temperatures for a specific metallic. In alloys, this rearrangement may also purpose an detail to be able to now no longer generally dissolve into the bottom metallic to abruptly emerge as soluble, even as a reversal of the allotropy will make the factors both partly or absolutely insoluble. When withinside the soluble state, the procedure of diffusion reasons the atoms of the dissolved detail to unfold out, trying to shape a homogenous distribution in the crystals of the bottom metallic.
- If the alloy is cooled to an insoluble state, the atoms of the dissolved constituents (solutes) may also migrate out of the answer. This kind of diffusion, referred to as precipitation, results in nucleation, wherein the migrating atoms organization collectively on the grain-barriers. This bureaucracy a microstructure usually which includes or greater wonderful phases. For instance, metal that has been heated above the austenizing temperature (crimson to orange-hot, or round 1,500 °F (820 °C) to 1,600 °F (870 °C) relying on carbon content), after which cooled slowly, bureaucracy a laminated shape composed of alternating layers of ferrite and cementite, turning into smooth pearlite. After heating the metal to the austenite section after which quenching it in water, the microstructure can be withinside the martensitic section.
- This is because of the truth that the metal will alternate from the austenite section to the martensite section after quenching. Some pearlite or ferrite can be gift if the quench did now no longer swiftly cool off all of the metal. Unlike iron-primarily based totally alloys, maximum warmness-treatable alloys do now no longer enjoy a ferrite transformation. In those alloys, the nucleation on the grain-barriers regularly reinforces the shape of the crystal matrix. These metals harden with the aid of using precipitation. Typically a sluggish procedure, relying on temperature, that is regularly noted as "age hardening". Many metals and non-metals showcase a martensite transformation whilst cooled quickly (with outside media like oil, polymer, water, etc.). When a metallic is cooled very quickly, the insoluble atoms won't be capable of migrate out of the answer in time. This is referred to as a "diffusionless transformation."
- When the crystal matrix adjustments to its low-temperature arrangement, the atoms of the solute emerge as trapped in the lattice. The trapped atoms save you the crystal matrix from absolutely converting into its low-temperature allotrope, developing shearing stresses in the lattice.
- When a few alloys are cooled quickly, along with metal, the martensite transformation hardens the metallic, even as in others, like aluminum, the alloy will become softer.
Q9) Define austempering.
A9)
- It is very similar to martempering. Steel is austenitized and then quenched in a salt bath maintained at a constant temperature in the range of 〖260-400〗^0 C.
- The article is held at this temperature for long enough to allow isothermal transformation to be completed.
- After the complete transformation of austenite to bainite, steel is cooled to room temperature in air. It is also called isothermal quenching.
- The temperature of quenching lies below the nose of the TTT curve and above the M_s temperature.
Q10) What is martempering?
A10)
- This is a hardening method that produces martensite. This method is also known as hardening by interrupted quenching
- First the steel is heated to the hardening temperature then quenched in a medium (salt bath) having a temperature slightly above the point where martensite starts to form (usually from 〖150-300〗^0 C.
- It is held until it reaches the temperature of the medium and then cooled further to room temperature in air or oil. The holding time in quenching medium or bath should be sufficient to enable a uniform temperature to be reduced throughout the cross section but not long enough to cause austenite decomposition.
- Austenite is transformed into martensite during the subsequent period of cooling to room temperature.
Q11) Explain patenting.
A11)
- A patent is a sort of highbrow assets that offers its proprietor the criminal proper to exclude others from making, using, or promoting an invention for a restricted time frame in alternate for publishing an permitting disclosure of the invention.
- In maximum nations, patent rights fall beneathneath non-public regulation and the patent holder need to sue a person infringing the patent so that it will put in force their rights. In a few industries patents are an important shape of aggressive advantage; in others they're irrelevant.
- The technique for granting patents, necessities positioned at the patentee, and the quantity of the unique rights range broadly among nations in keeping with countrywide legal guidelines and global agreements.
- Typically, however, a patent utility need to encompass one or greater claims that outline the scope of safety this is being sought.
- A patent may also encompass many claims, every of which defines a selected assets proper. These claims need to meet numerous patentability necessities, which withinside the US encompass novelty, usefulness, and non-obviousness. Under the World Trade Organization's (WTO) TRIPS Agreement, patents have to be to be had in WTO member states for any invention, in all fields of technology, furnished they're new, contain an imaginitive step, and are able to business utility.
- Nevertheless, there are versions on what's patentable situation count from usa to usa, additionally amongst WTO member states. TRIPS additionally affords that the time period of safety to be had have to be not less than twenty years.
Q12) What is retention of austenite?
A12)
- The phenomenon of retained austenite stabilization in device steels is nicely documented. When a metallic with a martensite end temperature (Mf) under room temperature is quenched, a few austenite is retained withinside the microstructure.
- If cooling is straight away persevered to a temperature under the Mf, surely all of the austenite gift at room temperature may be converted to martensite. However, if there's a postpone among quenching to room temperature and the similarly cooling, the austenite can stabilize and can't then be converted through next cooling .
- It is critical to understand if this phenomenon additionally applies to carburized low alloy steels used to make gears. As nicely as lowering hardness and put on resistance, retained austenite withinside the case of such additives can later be converted through implemented stress, inflicting distortion in the course of service.
- Retained austenite has additionally been pronounced to result in cracking in the course of grinding after warmth treatment.
- There isn't anyt any settlement withinside the literature at the prevalence of stabilization, with a few reviews suggesting that it does now no longer arise in carburized low alloy steels . Others, however, file that it does arise and a few even endorse that its onset could be very rapid .
- Where stabilization is pronounced, it's miles normally related to excessive alloy content, mainly nickel, and with the presence of nitrogen withinside the case. The paintings pronounced right here set out to reveal the results of stabilization on a normal carburizing metallic (SAE 8620) after carburizing in a normal business cycle with none delivered nitrogen.
Q13) What is tempering?
A13)
- The tempering process provides a method for transforming martensite into ferrite and cementite. How much of the martensite is transformed depends on the temperature and time of the tempering process.
- Tempering is a heat treatment process that alters the mechanical properties (typically ductility and hardness) and relieves internal stresses of a steel. Tempering allows carbon trapped in a martensitic microstructure to disperse, and enables the internal stresses to be released from the steel that may have been created from prior operations.
The Tempering Process
- Tempering is performed by elevating the steel to a set point below its lower critical temperature, typically following a hardening operation. Once this temperature is reached, it is held there for a specified amount of time. The exact temperature and time depend on several factors such as the type of steel and desired mechanical properties.
- To get the steel to its critical temperature, some type of heating device must be used. Common devices include gas furnaces, electrical resistance furnaces, or induction furnaces. Often, this heating is done in a vacuum or with an inert gas to protect the steel from oxidation. Once the furnace achieves the desired temperature, a dwell time occurs. Following the dwell time, the furnace is shut off and the steel is allowed to cool at predetermined rate.
Why Is Steel Tempered?
- Tempering steel after a hardening process allows for a middle ground of hardness and strength. This is achieved by allowing the carbon diffusion to occur within a steel microstructure. When steel is hardened, it can become excessively brittle and hard. However, when not hardened, the steel may not have the strength or abrasion resistance needed for its intended application. Tempering also improves the machinability and formability of a hardened steel, and can reduce the risk of the steel cracking or failing due to internal stresses.
When Is Tempering Used?
- Tempering is most commonly used following a quenching operation. Heating a carbon steel and rapidly quenching it can leave it too hard and brittle. Tempering it can restore some of its ductility.
- Tempering can reduce the hardness and relieve the stress of a welded component. Welds can create a localized zone that has been hardened due to the heat of the welding process. This can leave undesirable mechanical properties and residual stress that can promote hydrogen cracking. Tempering helps prevent this.
- Work hardened materials often require tempering. Materials can become work hardened through processes such as punching, bending, forming, drilling, or rolling. Work hardened materials have a high amount of residual stresses that can be alleviated through a tempering process
Q14) Explain flame and Induction hardening.
A14)
- Induction hardening includes the use of brought about electric currents to very unexpectedly generate warmth through hysteresis, typically in a workpiece crafted from medium to excessive carbon steel.
- Flame hardening makes use of oxy-gasoline burners to warmth the workpiece through conduction. Both methods use quenching after heating, regularly observed through tempering and/or pressure relieving.
- Induction hardening Surface of thing heated via way of means of excessive frequency induction, the frequency and energy necessities relying on length and geometry of thing and intensity of hardening required.
- Three essential kinds of induction machines: vacuum tube oscillator > 500 kHz spark hole oscillator 10–30 kHz motor generator 5–10 kHz Heat-up instances may be managed to within ±0.1 s and have to be much less than 20 s to save you distortion and keep away from post-remedy machining or grinding. Process also can be used for via hardening of small components. Tempering and/or pressure relieving also can be performed via way of means of induction heating, regularly with the identical induction coil.
- Process is without difficulty automatic and may deliver uniform high-satisfactory and repeatability, even though excessive capital and going for walks charges make it maximum appropriate for lengthy manufacturing runs. Typical packages encompass gears, crankshafts and camshafts.
- Materials: Ferrous metals whose composition is such that the specified hardness might be acquired on quenching from the austenite region: at the least 0.3% C. Most substances have as a minimum 0.4% C, and as much as 0.7% C. Plain carbon steels, alloy steels and grey, nodular and malleable solid irons; the temperature relies upon at the material: 750–760° C 0.5% undeniable carbon 900–800° C chromium–molybdenum alloy steels 950–980°C nodular iron Alloy steels, despite the fact that extra expensive, produce extra case depths.
- The better the carbon content, the extra the threat of cracks and/or distortion. Flame hardening isn't always generally appropriate for steels with middle strengths of extra than 900 MPa. Required middle electricity need to be acquired through suitable warmness remedy earlier than hardening.
- To permit carbides to enter answer at some stage in the fast heating times, the carbides must be small, for this reason normalised systems are preferred. Fine grains deliver higher manage over the case intensity and much less hazard of cracking and/or distortion. Very little grain grown occurs, because of quick heating times.
- Little or no decarburising or oxidation because of quick heating times.
- Surface hardnesses depend upon composition, temperature and quenchant, however are generally withinside the variety 350–seven-hundred Hv.
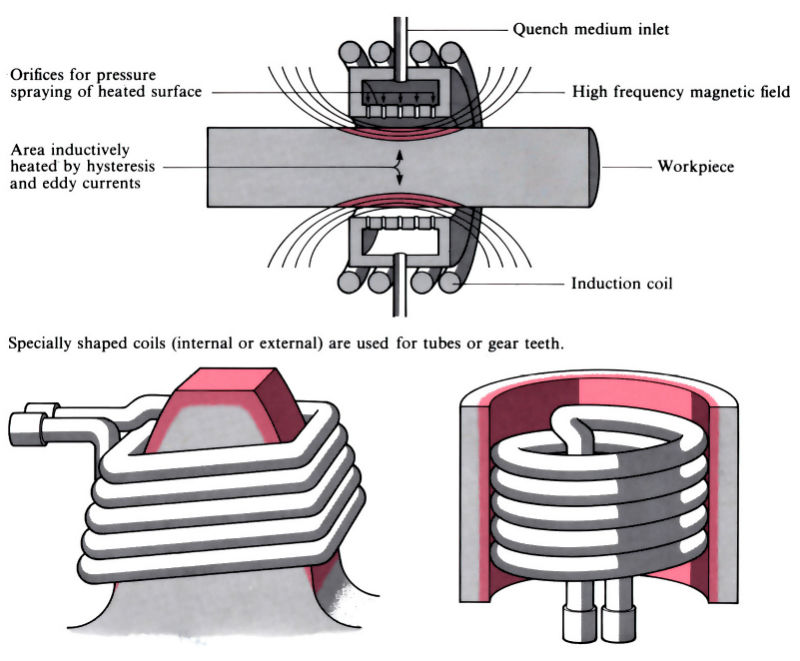
Fig: Flame and Induction hardening
Unit - 3
Heat treatment and its importance
Q1) Explain heat treatment and its importance in detail.
A1)
- All heating processes are almost similar because all the processes involve heating and cooling. The things which makes difference in it is cooling temperature or say cooling rate, heating temperature and also quenching medium to achieve desired properties.
- The heat treatment of ferrous metals (metals with iron) usually consist of annealing, normalizing and tempering.
- Most nonferrous metals can be annealed but never tempered, normalized for case hardened.
Heat treatment process: -
1) Hardening- Hardness is usually followed by tempering to minimize brittleness. Steel hardening is carried out by heating to temperature above critical temperature.
- Done quenching in water, oil.
- Final microstructure is martensite.
2) Tempering: - After hardening tempering is done.as we get hard and brittle structure while hardening so after secondary heating of martensite is done to get desired ductility, hardness and strength.
In tempering ductility increases with slight loss in hardness and steel.
3) Annealing: -
- Annealing involves heating the material to predetermined temperature and hold the material at the temperature and cool at room temperature i.e. slowly. It helps to relieve internal stresses, improve ductility and toughness, refrain grain size, enhance machinability.
Different types of annealing processes: -
a) Full annealing- In full annealing steel is heated above upper critical temperature i.e. 723°C. Heating will be done nearly at 800°C i.e. 50-70°C above the critical temperature depending upon the carbon percentage.
After heating we will cool at very slowly then it will become ductile in case of hypoeutectoid Steel and in hypereutectoid we will get cementite + pearlite.
After full annealing machinability increases and becomes more ductile.
b) Process annealing: - Done at 550-650°C done below lower critical temperature.
In hypoeutectoid steel (where carbon percentage is less than 0.8%) and hypereutectoid steel (carbon percentage more than 0.8%) in both cases heating done below lower critical temperature. It is done to improve refrain grain size and improves ductility.
c) Spherodise annealing: - It is done on the metals which are very hard to machine. It is done at lower critical temperature i.e. 723-770°C. Here we heat steel and then do slowly cooling by which carbide forms sphere structure. It is mainly done to enhance machinability which are very hard to do machining.
d) Isothermal annealing: - It is very much similar to full annealing process. But the major difference in it is that in case of full annealing hypoeutectoid and hypereutectoid can be done but isothermal annealing is done only add hypoeutectoid Steel.
Heating is done above 723 degree Celsius. It is done to reduce annealing time and to produce homogeneous structure in the material and also to improve machinability.
e) Stress relieving annealing: - As name suggest, this process is done to relieve internal stresses. No microstructural changes occurred during the process.
It is done at lower critical temperature followed by a uniform cooling.
4) Normalizing: -
Heated beyond upper critical temperature and cooled in still air. Soap rate of cooling gets increased. Get harder and structure than annealing.
5) Austempering: - Heating above critical temperature to make it austenitic. Then it is quenched at critical rate in to Salt but held at Bainite range (200-420°C). Austenite is completely transformed into Bainite then cooled at room temperature.
6) Martempering: - Heated above critical range to make it into Austenite and then quenched into salt bath and maintaining temperature for long time during transformation so that there is least internal stresses and distortion. They are cooled in air to room temperature.
7) Sub-zero Treatment: -Sub-zero treatment are treatments in which components are cooled below room temperature. There can be many reasons, but the main reason is to remove retained austenite from quenched components, to increase the wear resistance or to stabilise the component. It is carried out in order to complete the transformation of retained austenite to martensite after hardening and before tempering. It is usually applied to high carbon, high alloy steel but is more widely applied by aerospace companies.
Q2) Explain hardenability.
A2)
Hardenability:
- The hardenability of a metal alloy is the depth to which a material is hardened after putting it through a heat treatment process.
- It should not be confused hardness which is a measure of samples resistance to indentation or scratching.
- It is an important property of welding since it is inversely proportional to weldability that is the ease of welding a material. Hardenability is a term that is used to describe a given steels ability to harden it doesn't mean what hardness can be achieved.
- Hardness is dependent mostly on a ferrous material’s carbon content. The major factor which affects hardenability and the rate of austenite transformation are carbon content, grain size and alloying element.
Q3) Explain annealing
A3)
- When a material is plastically strained the yield, stress is increased. In many of the manufacturing techniques listed earlier, the material is plastically deformed in order to fabricate the desired shape.Aalthough this may significantly increase the yield strength of the material, it also makes it more brittle.
- This change is a result of the dislocation density, or imperfections in the lattice, increasing as a result of the deformation.
- If we take a work-hardened material and subject it to a sufficiently high temperature for a specified time, we can reduce the dislocation density and the yield stress will return to its initial value.
- This is known as annealing the material, which is a form of heat treatment. Due to other aspects, such as recrystallization, subjecting the work-hardened material to a high temperature for too long
Q4) What is normalizing
A4)
- Steels that have undergo plastic deformation consist of pearlites which are irregularly shaped and relatively large, but varying in size. Normalizing is a heat treatment used on steel so as to refine its crystal structure and produces a more uniform and desired grain size distribution. Fine grained pearlites are tougher than coarse grained ones.
- Normalization eliminates internal stresses, strains and improves the mechanical properties of the steel, such as improving its toughness and machinability. A better ductility can also be obtained without compromising the hardness and strength.
- Normalizing is accomplished by heating the steel to a temperature above the transformation range and into the range of complete austenite. This is dependent on the composition of the steel as indicated by the iron-carbon diagram shown below.
- The usual normalizing temperature ranges from 815°C to 980°C (1500°F to 1800°F), depending on the steel involved.
- After sufficient time is given for complete transformation to austenite, i.e., austenitizing of the steel, the alloy is air-cooled to a temperature substantially below the transformation range. The air-cooling avoids excessive proeutectoid segregation. The cooling rate is usually in the range of 500 to 1000 °C/h (900 to 1800 °F/h).
- The final microstructure consists of fine pearlite and an absence of massive proeutectoid ferrite. Normalizing is commonly specified for plates of pressure vessel quality hence to ASTM standards above 1 ½ inch in thickness
Q5) What is hardening?
A5)
- The development of extremely small uniformly dispersed particles of a second phase within the original phase matrix can improve the strength and hardness of particular metal alloys; this must be accomplished by phase transformations induced by proper heat treatments.
- Precipitation hardening is the name given to the process since the new phase's microscopic particles are called precipitates. The term "age hardening" is also used to describe this operation because the alloy's strength increases over time.
- Aluminum–copper, copper–beryllium, copper–tin, and magnesium–aluminum alloys are examples of precipitation hardenable alloys; some ferrous alloys are also precipitation hardenable.
- Even though the heat treatment procedures are similar, precipitation hardening and the treatment of steel to generate tempered martensite are two distinct phenomena that should not be conflated. The main distinction is in the processes used to accomplish hardening and strengthening. As precipitation hardening is explained, they should become clear.
Q6) What is quench cracks?
A6)
- Quench cracks end result from stresses produced at some point of the transition from Austenite to Martensite, which includes an growth in quantity. The martensitic transformation begins offevolved on the outermost surfaces of the element being quenched.
- As the transformation is going deeper into the softer austenite closer to middle of mass, its alternate in quantity is limited via way of means of the martensite already created withinside the outer volumes of the element adjoining to the floor.
- This creates inner stresses which region the floor into tension. When sufficient martensite has shaped to create inner strain extra than the last strength (tensile strength) of the as quenched martensite on the floor, a crack results. As-quenched Martensite is difficult and brittle- it has genuinely no ductility.
Q7) Explain hardenability test
A7)
- Hardenability is a measure that relates the effect of alloy composition on a steel alloy's ability to transition to martensite for a specific quenching procedure. There is a unique relationship between mechanical qualities and cooling rate for each distinct steel alloy.
- The ability of an alloy to be hardened by the development of martensite as a result of a specific heat treatment is referred to as hardenability.
- Hardenability is a qualitative measure of the rate at which hardness drops down with distance into the interior of a specimen as a result of decreasing martensite content, rather than "hardness," which is the resistance to indentation. A steel alloy with a high hardenability hardens, or develops martensite, not only on the surface but also throughout the inside to a great extent.
Q8) Explain TTT diagram and its construction and related Heat Treatment Processes.
A8)
TTT diagram:
- T-T-T diagram is likewise known as isothermal transformation diagram [Temperature-Time –Transformation]. It is a plot of temperature as opposed to the logarithm of time for a metallic alloy of specific composition.
- It is used to decide while ameliorations start and cease for an isothermal [constant thermal] warmth remedy of a formerly austenitized alloy.
- Time-Temperature-Transformation (TTT) diagram or S-curve refers to simplest one metallic of a selected composition at a time, which applies to all carbon steels. This diagram is likewise referred to as as C-curve isothermal (decomposition of austenite) diagram and Bain’s curve.
- The impact of time-temperature at the microstructure adjustments of metallic may be proven through the TTT diagram.
- These diagrams are significantly used withinside the evaluation of the decomposition of austenite in warmth-treatable steels. We have visible that the iron-carbon section diagram does now no longer display time as a variable and subsequently the outcomes of various cooling prices at the systems of steels aren't revealed. Moreover, equilibrium situations aren't maintained in warmth treatment.
- Although, the iron-carbon equilibrium diagram well-knownshows at the stages and corresponding microstructures below equilibrium situations however numerous beneficial residences of the steels may be acquired below non-equilibrium situations, e.g. Variable prices of cooling as produced at some point of quenching and higher transformation of austenite into pearlite and martensite.
Construction:
- For every metallic composition, special IT diagram is acquired. Fig suggests the TTT diagram of eutectoid metallic (i.e. metallic containing 0.8% C). Austenite is strong above eutectoid temperature 727 °C. When metallic is cooled to a temperature beneathneath this eutectoid temperature, austenite is converted into its transformation product.
- TTT diagram relates the transformation of austenite to time and temperature situations. Thus, the TTT diagram suggests transformation merchandise in keeping with temperature and additionally the time required for entire transformation. Curve 1 is transformation start curve even as curve 2 is the transformation quit curve.
- The location to the left of curve 1 corresponds to austenite (A’). The location to the proper of curve 2 represents the entire transformation of austenite (F+C). The c programming language among those curves suggests partial decomposition of austenite into ferrite and Cementite (A’+F+C).
Heat treatment process:
- Heat treating (or warmness remedy) is a collection of industrial, thermal and metalworking procedures used to regulate the physical, and every so often chemical, homes of a material. The maximum not unusualplace utility is metallurgical. Heat remedies also are used withinside the manufacture of many different materials, inclusive of glass. Heat remedy entails using heating or chilling, typically to excessive temperatures, to obtain the preferred end result inclusive of hardening or softening of a material. Heat remedy strategies consist of annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching.
- Although the time period warmness remedy applies handiest to procedures in which the heating and cooling are performed for the unique motive of changing homes intentionally, heating and cooling frequently arise by the way throughout different production procedures inclusive of warm forming or welding.
- Metallic substances encompass a microstructure of small crystals referred to as "grains" or crystallites. The nature of the grains (i.e. grain length and composition) is one of the only elements that may decide the general mechanical conduct of the metallic. Heat remedy affords an green manner to control the houses of the metallic with the aid of using controlling the fee of diffusion and the fee of cooling in the microstructure. Heat treating is regularly used to adjust the mechanical houses of a metal alloy, manipulating houses along with the hardness, strength, toughness, ductility, and elasticity.
- There are mechanisms which can alternate an alloy's houses at some stage in warmness remedy: the formation of martensite reasons the crystals to deform intrinsically, and the diffusion mechanism reasons adjustments withinside the homogeneity of the alloy. The crystal shape includes atoms which might be grouped in a completely particular arrangement, referred to as a lattice. In maximum factors, this order will rearrange itself, relying on situations like temperature and pressure.
- This rearrangement referred to as allotropy or polymorphism, may also arise numerous times, at many unique temperatures for a specific metallic. In alloys, this rearrangement may also purpose an detail to be able to now no longer generally dissolve into the bottom metallic to abruptly emerge as soluble, even as a reversal of the allotropy will make the factors both partly or absolutely insoluble. When withinside the soluble state, the procedure of diffusion reasons the atoms of the dissolved detail to unfold out, trying to shape a homogenous distribution in the crystals of the bottom metallic.
- If the alloy is cooled to an insoluble state, the atoms of the dissolved constituents (solutes) may also migrate out of the answer. This kind of diffusion, referred to as precipitation, results in nucleation, wherein the migrating atoms organization collectively on the grain-barriers. This bureaucracy a microstructure usually which includes or greater wonderful phases. For instance, metal that has been heated above the austenizing temperature (crimson to orange-hot, or round 1,500 °F (820 °C) to 1,600 °F (870 °C) relying on carbon content), after which cooled slowly, bureaucracy a laminated shape composed of alternating layers of ferrite and cementite, turning into smooth pearlite. After heating the metal to the austenite section after which quenching it in water, the microstructure can be withinside the martensitic section.
- This is because of the truth that the metal will alternate from the austenite section to the martensite section after quenching. Some pearlite or ferrite can be gift if the quench did now no longer swiftly cool off all of the metal. Unlike iron-primarily based totally alloys, maximum warmness-treatable alloys do now no longer enjoy a ferrite transformation. In those alloys, the nucleation on the grain-barriers regularly reinforces the shape of the crystal matrix. These metals harden with the aid of using precipitation. Typically a sluggish procedure, relying on temperature, that is regularly noted as "age hardening". Many metals and non-metals showcase a martensite transformation whilst cooled quickly (with outside media like oil, polymer, water, etc.). When a metallic is cooled very quickly, the insoluble atoms won't be capable of migrate out of the answer in time. This is referred to as a "diffusionless transformation."
- When the crystal matrix adjustments to its low-temperature arrangement, the atoms of the solute emerge as trapped in the lattice. The trapped atoms save you the crystal matrix from absolutely converting into its low-temperature allotrope, developing shearing stresses in the lattice.
- When a few alloys are cooled quickly, along with metal, the martensite transformation hardens the metallic, even as in others, like aluminum, the alloy will become softer.
Q9) Define austempering.
A9)
- It is very similar to martempering. Steel is austenitized and then quenched in a salt bath maintained at a constant temperature in the range of 〖260-400〗^0 C.
- The article is held at this temperature for long enough to allow isothermal transformation to be completed.
- After the complete transformation of austenite to bainite, steel is cooled to room temperature in air. It is also called isothermal quenching.
- The temperature of quenching lies below the nose of the TTT curve and above the M_s temperature.
Q10) What is martempering?
A10)
- This is a hardening method that produces martensite. This method is also known as hardening by interrupted quenching
- First the steel is heated to the hardening temperature then quenched in a medium (salt bath) having a temperature slightly above the point where martensite starts to form (usually from 〖150-300〗^0 C.
- It is held until it reaches the temperature of the medium and then cooled further to room temperature in air or oil. The holding time in quenching medium or bath should be sufficient to enable a uniform temperature to be reduced throughout the cross section but not long enough to cause austenite decomposition.
- Austenite is transformed into martensite during the subsequent period of cooling to room temperature.
Q11) Explain patenting.
A11)
- A patent is a sort of highbrow assets that offers its proprietor the criminal proper to exclude others from making, using, or promoting an invention for a restricted time frame in alternate for publishing an permitting disclosure of the invention.
- In maximum nations, patent rights fall beneathneath non-public regulation and the patent holder need to sue a person infringing the patent so that it will put in force their rights. In a few industries patents are an important shape of aggressive advantage; in others they're irrelevant.
- The technique for granting patents, necessities positioned at the patentee, and the quantity of the unique rights range broadly among nations in keeping with countrywide legal guidelines and global agreements.
- Typically, however, a patent utility need to encompass one or greater claims that outline the scope of safety this is being sought.
- A patent may also encompass many claims, every of which defines a selected assets proper. These claims need to meet numerous patentability necessities, which withinside the US encompass novelty, usefulness, and non-obviousness. Under the World Trade Organization's (WTO) TRIPS Agreement, patents have to be to be had in WTO member states for any invention, in all fields of technology, furnished they're new, contain an imaginitive step, and are able to business utility.
- Nevertheless, there are versions on what's patentable situation count from usa to usa, additionally amongst WTO member states. TRIPS additionally affords that the time period of safety to be had have to be not less than twenty years.
Q12) What is retention of austenite?
A12)
- The phenomenon of retained austenite stabilization in device steels is nicely documented. When a metallic with a martensite end temperature (Mf) under room temperature is quenched, a few austenite is retained withinside the microstructure.
- If cooling is straight away persevered to a temperature under the Mf, surely all of the austenite gift at room temperature may be converted to martensite. However, if there's a postpone among quenching to room temperature and the similarly cooling, the austenite can stabilize and can't then be converted through next cooling .
- It is critical to understand if this phenomenon additionally applies to carburized low alloy steels used to make gears. As nicely as lowering hardness and put on resistance, retained austenite withinside the case of such additives can later be converted through implemented stress, inflicting distortion in the course of service.
- Retained austenite has additionally been pronounced to result in cracking in the course of grinding after warmth treatment.
- There isn't anyt any settlement withinside the literature at the prevalence of stabilization, with a few reviews suggesting that it does now no longer arise in carburized low alloy steels . Others, however, file that it does arise and a few even endorse that its onset could be very rapid .
- Where stabilization is pronounced, it's miles normally related to excessive alloy content, mainly nickel, and with the presence of nitrogen withinside the case. The paintings pronounced right here set out to reveal the results of stabilization on a normal carburizing metallic (SAE 8620) after carburizing in a normal business cycle with none delivered nitrogen.
Q13) What is tempering?
A13)
- The tempering process provides a method for transforming martensite into ferrite and cementite. How much of the martensite is transformed depends on the temperature and time of the tempering process.
- Tempering is a heat treatment process that alters the mechanical properties (typically ductility and hardness) and relieves internal stresses of a steel. Tempering allows carbon trapped in a martensitic microstructure to disperse, and enables the internal stresses to be released from the steel that may have been created from prior operations.
The Tempering Process
- Tempering is performed by elevating the steel to a set point below its lower critical temperature, typically following a hardening operation. Once this temperature is reached, it is held there for a specified amount of time. The exact temperature and time depend on several factors such as the type of steel and desired mechanical properties.
- To get the steel to its critical temperature, some type of heating device must be used. Common devices include gas furnaces, electrical resistance furnaces, or induction furnaces. Often, this heating is done in a vacuum or with an inert gas to protect the steel from oxidation. Once the furnace achieves the desired temperature, a dwell time occurs. Following the dwell time, the furnace is shut off and the steel is allowed to cool at predetermined rate.
Why Is Steel Tempered?
- Tempering steel after a hardening process allows for a middle ground of hardness and strength. This is achieved by allowing the carbon diffusion to occur within a steel microstructure. When steel is hardened, it can become excessively brittle and hard. However, when not hardened, the steel may not have the strength or abrasion resistance needed for its intended application. Tempering also improves the machinability and formability of a hardened steel, and can reduce the risk of the steel cracking or failing due to internal stresses.
When Is Tempering Used?
- Tempering is most commonly used following a quenching operation. Heating a carbon steel and rapidly quenching it can leave it too hard and brittle. Tempering it can restore some of its ductility.
- Tempering can reduce the hardness and relieve the stress of a welded component. Welds can create a localized zone that has been hardened due to the heat of the welding process. This can leave undesirable mechanical properties and residual stress that can promote hydrogen cracking. Tempering helps prevent this.
- Work hardened materials often require tempering. Materials can become work hardened through processes such as punching, bending, forming, drilling, or rolling. Work hardened materials have a high amount of residual stresses that can be alleviated through a tempering process
Q14) Explain flame and Induction hardening.
A14)
- Induction hardening includes the use of brought about electric currents to very unexpectedly generate warmth through hysteresis, typically in a workpiece crafted from medium to excessive carbon steel.
- Flame hardening makes use of oxy-gasoline burners to warmth the workpiece through conduction. Both methods use quenching after heating, regularly observed through tempering and/or pressure relieving.
- Induction hardening Surface of thing heated via way of means of excessive frequency induction, the frequency and energy necessities relying on length and geometry of thing and intensity of hardening required.
- Three essential kinds of induction machines: vacuum tube oscillator > 500 kHz spark hole oscillator 10–30 kHz motor generator 5–10 kHz Heat-up instances may be managed to within ±0.1 s and have to be much less than 20 s to save you distortion and keep away from post-remedy machining or grinding. Process also can be used for via hardening of small components. Tempering and/or pressure relieving also can be performed via way of means of induction heating, regularly with the identical induction coil.
- Process is without difficulty automatic and may deliver uniform high-satisfactory and repeatability, even though excessive capital and going for walks charges make it maximum appropriate for lengthy manufacturing runs. Typical packages encompass gears, crankshafts and camshafts.
- Materials: Ferrous metals whose composition is such that the specified hardness might be acquired on quenching from the austenite region: at the least 0.3% C. Most substances have as a minimum 0.4% C, and as much as 0.7% C. Plain carbon steels, alloy steels and grey, nodular and malleable solid irons; the temperature relies upon at the material: 750–760° C 0.5% undeniable carbon 900–800° C chromium–molybdenum alloy steels 950–980°C nodular iron Alloy steels, despite the fact that extra expensive, produce extra case depths.
- The better the carbon content, the extra the threat of cracks and/or distortion. Flame hardening isn't always generally appropriate for steels with middle strengths of extra than 900 MPa. Required middle electricity need to be acquired through suitable warmness remedy earlier than hardening.
- To permit carbides to enter answer at some stage in the fast heating times, the carbides must be small, for this reason normalised systems are preferred. Fine grains deliver higher manage over the case intensity and much less hazard of cracking and/or distortion. Very little grain grown occurs, because of quick heating times.
- Little or no decarburising or oxidation because of quick heating times.
- Surface hardnesses depend upon composition, temperature and quenchant, however are generally withinside the variety 350–seven-hundred Hv.
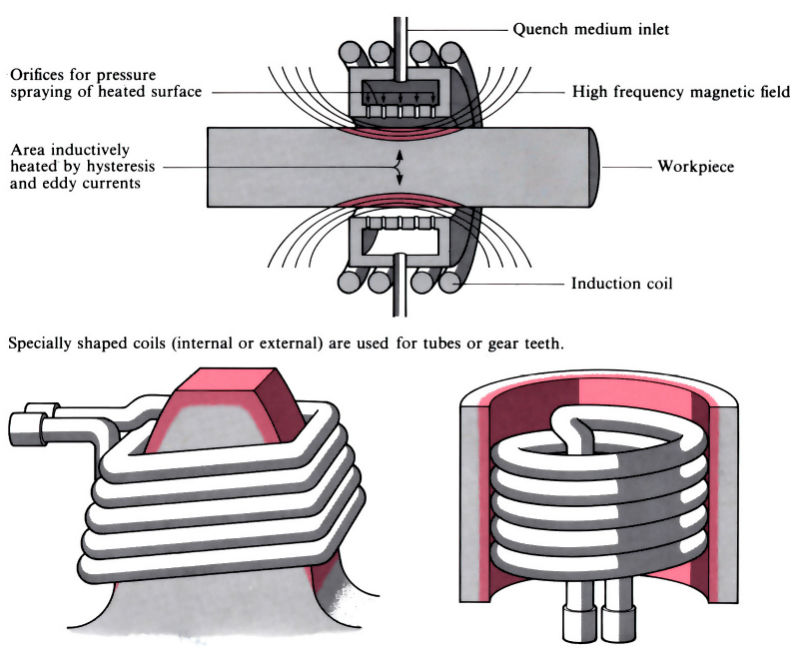
Fig: Flame and Induction hardening
Unit - 3
Heat treatment and its importance
Q1) Explain heat treatment and its importance in detail.
A1)
- All heating processes are almost similar because all the processes involve heating and cooling. The things which makes difference in it is cooling temperature or say cooling rate, heating temperature and also quenching medium to achieve desired properties.
- The heat treatment of ferrous metals (metals with iron) usually consist of annealing, normalizing and tempering.
- Most nonferrous metals can be annealed but never tempered, normalized for case hardened.
Heat treatment process: -
1) Hardening- Hardness is usually followed by tempering to minimize brittleness. Steel hardening is carried out by heating to temperature above critical temperature.
- Done quenching in water, oil.
- Final microstructure is martensite.
2) Tempering: - After hardening tempering is done.as we get hard and brittle structure while hardening so after secondary heating of martensite is done to get desired ductility, hardness and strength.
In tempering ductility increases with slight loss in hardness and steel.
3) Annealing: -
- Annealing involves heating the material to predetermined temperature and hold the material at the temperature and cool at room temperature i.e. slowly. It helps to relieve internal stresses, improve ductility and toughness, refrain grain size, enhance machinability.
Different types of annealing processes: -
a) Full annealing- In full annealing steel is heated above upper critical temperature i.e. 723°C. Heating will be done nearly at 800°C i.e. 50-70°C above the critical temperature depending upon the carbon percentage.
After heating we will cool at very slowly then it will become ductile in case of hypoeutectoid Steel and in hypereutectoid we will get cementite + pearlite.
After full annealing machinability increases and becomes more ductile.
b) Process annealing: - Done at 550-650°C done below lower critical temperature.
In hypoeutectoid steel (where carbon percentage is less than 0.8%) and hypereutectoid steel (carbon percentage more than 0.8%) in both cases heating done below lower critical temperature. It is done to improve refrain grain size and improves ductility.
c) Spherodise annealing: - It is done on the metals which are very hard to machine. It is done at lower critical temperature i.e. 723-770°C. Here we heat steel and then do slowly cooling by which carbide forms sphere structure. It is mainly done to enhance machinability which are very hard to do machining.
d) Isothermal annealing: - It is very much similar to full annealing process. But the major difference in it is that in case of full annealing hypoeutectoid and hypereutectoid can be done but isothermal annealing is done only add hypoeutectoid Steel.
Heating is done above 723 degree Celsius. It is done to reduce annealing time and to produce homogeneous structure in the material and also to improve machinability.
e) Stress relieving annealing: - As name suggest, this process is done to relieve internal stresses. No microstructural changes occurred during the process.
It is done at lower critical temperature followed by a uniform cooling.
4) Normalizing: -
Heated beyond upper critical temperature and cooled in still air. Soap rate of cooling gets increased. Get harder and structure than annealing.
5) Austempering: - Heating above critical temperature to make it austenitic. Then it is quenched at critical rate in to Salt but held at Bainite range (200-420°C). Austenite is completely transformed into Bainite then cooled at room temperature.
6) Martempering: - Heated above critical range to make it into Austenite and then quenched into salt bath and maintaining temperature for long time during transformation so that there is least internal stresses and distortion. They are cooled in air to room temperature.
7) Sub-zero Treatment: -Sub-zero treatment are treatments in which components are cooled below room temperature. There can be many reasons, but the main reason is to remove retained austenite from quenched components, to increase the wear resistance or to stabilise the component. It is carried out in order to complete the transformation of retained austenite to martensite after hardening and before tempering. It is usually applied to high carbon, high alloy steel but is more widely applied by aerospace companies.
Q2) Explain hardenability.
A2)
Hardenability:
- The hardenability of a metal alloy is the depth to which a material is hardened after putting it through a heat treatment process.
- It should not be confused hardness which is a measure of samples resistance to indentation or scratching.
- It is an important property of welding since it is inversely proportional to weldability that is the ease of welding a material. Hardenability is a term that is used to describe a given steels ability to harden it doesn't mean what hardness can be achieved.
- Hardness is dependent mostly on a ferrous material’s carbon content. The major factor which affects hardenability and the rate of austenite transformation are carbon content, grain size and alloying element.
Q3) Explain annealing
A3)
- When a material is plastically strained the yield, stress is increased. In many of the manufacturing techniques listed earlier, the material is plastically deformed in order to fabricate the desired shape.Aalthough this may significantly increase the yield strength of the material, it also makes it more brittle.
- This change is a result of the dislocation density, or imperfections in the lattice, increasing as a result of the deformation.
- If we take a work-hardened material and subject it to a sufficiently high temperature for a specified time, we can reduce the dislocation density and the yield stress will return to its initial value.
- This is known as annealing the material, which is a form of heat treatment. Due to other aspects, such as recrystallization, subjecting the work-hardened material to a high temperature for too long
Q4) What is normalizing
A4)
- Steels that have undergo plastic deformation consist of pearlites which are irregularly shaped and relatively large, but varying in size. Normalizing is a heat treatment used on steel so as to refine its crystal structure and produces a more uniform and desired grain size distribution. Fine grained pearlites are tougher than coarse grained ones.
- Normalization eliminates internal stresses, strains and improves the mechanical properties of the steel, such as improving its toughness and machinability. A better ductility can also be obtained without compromising the hardness and strength.
- Normalizing is accomplished by heating the steel to a temperature above the transformation range and into the range of complete austenite. This is dependent on the composition of the steel as indicated by the iron-carbon diagram shown below.
- The usual normalizing temperature ranges from 815°C to 980°C (1500°F to 1800°F), depending on the steel involved.
- After sufficient time is given for complete transformation to austenite, i.e., austenitizing of the steel, the alloy is air-cooled to a temperature substantially below the transformation range. The air-cooling avoids excessive proeutectoid segregation. The cooling rate is usually in the range of 500 to 1000 °C/h (900 to 1800 °F/h).
- The final microstructure consists of fine pearlite and an absence of massive proeutectoid ferrite. Normalizing is commonly specified for plates of pressure vessel quality hence to ASTM standards above 1 ½ inch in thickness
Q5) What is hardening?
A5)
- The development of extremely small uniformly dispersed particles of a second phase within the original phase matrix can improve the strength and hardness of particular metal alloys; this must be accomplished by phase transformations induced by proper heat treatments.
- Precipitation hardening is the name given to the process since the new phase's microscopic particles are called precipitates. The term "age hardening" is also used to describe this operation because the alloy's strength increases over time.
- Aluminum–copper, copper–beryllium, copper–tin, and magnesium–aluminum alloys are examples of precipitation hardenable alloys; some ferrous alloys are also precipitation hardenable.
- Even though the heat treatment procedures are similar, precipitation hardening and the treatment of steel to generate tempered martensite are two distinct phenomena that should not be conflated. The main distinction is in the processes used to accomplish hardening and strengthening. As precipitation hardening is explained, they should become clear.
Q6) What is quench cracks?
A6)
- Quench cracks end result from stresses produced at some point of the transition from Austenite to Martensite, which includes an growth in quantity. The martensitic transformation begins offevolved on the outermost surfaces of the element being quenched.
- As the transformation is going deeper into the softer austenite closer to middle of mass, its alternate in quantity is limited via way of means of the martensite already created withinside the outer volumes of the element adjoining to the floor.
- This creates inner stresses which region the floor into tension. When sufficient martensite has shaped to create inner strain extra than the last strength (tensile strength) of the as quenched martensite on the floor, a crack results. As-quenched Martensite is difficult and brittle- it has genuinely no ductility.
Q7) Explain hardenability test
A7)
- Hardenability is a measure that relates the effect of alloy composition on a steel alloy's ability to transition to martensite for a specific quenching procedure. There is a unique relationship between mechanical qualities and cooling rate for each distinct steel alloy.
- The ability of an alloy to be hardened by the development of martensite as a result of a specific heat treatment is referred to as hardenability.
- Hardenability is a qualitative measure of the rate at which hardness drops down with distance into the interior of a specimen as a result of decreasing martensite content, rather than "hardness," which is the resistance to indentation. A steel alloy with a high hardenability hardens, or develops martensite, not only on the surface but also throughout the inside to a great extent.
Q8) Explain TTT diagram and its construction and related Heat Treatment Processes.
A8)
TTT diagram:
- T-T-T diagram is likewise known as isothermal transformation diagram [Temperature-Time –Transformation]. It is a plot of temperature as opposed to the logarithm of time for a metallic alloy of specific composition.
- It is used to decide while ameliorations start and cease for an isothermal [constant thermal] warmth remedy of a formerly austenitized alloy.
- Time-Temperature-Transformation (TTT) diagram or S-curve refers to simplest one metallic of a selected composition at a time, which applies to all carbon steels. This diagram is likewise referred to as as C-curve isothermal (decomposition of austenite) diagram and Bain’s curve.
- The impact of time-temperature at the microstructure adjustments of metallic may be proven through the TTT diagram.
- These diagrams are significantly used withinside the evaluation of the decomposition of austenite in warmth-treatable steels. We have visible that the iron-carbon section diagram does now no longer display time as a variable and subsequently the outcomes of various cooling prices at the systems of steels aren't revealed. Moreover, equilibrium situations aren't maintained in warmth treatment.
- Although, the iron-carbon equilibrium diagram well-knownshows at the stages and corresponding microstructures below equilibrium situations however numerous beneficial residences of the steels may be acquired below non-equilibrium situations, e.g. Variable prices of cooling as produced at some point of quenching and higher transformation of austenite into pearlite and martensite.
Construction:
- For every metallic composition, special IT diagram is acquired. Fig suggests the TTT diagram of eutectoid metallic (i.e. metallic containing 0.8% C). Austenite is strong above eutectoid temperature 727 °C. When metallic is cooled to a temperature beneathneath this eutectoid temperature, austenite is converted into its transformation product.
- TTT diagram relates the transformation of austenite to time and temperature situations. Thus, the TTT diagram suggests transformation merchandise in keeping with temperature and additionally the time required for entire transformation. Curve 1 is transformation start curve even as curve 2 is the transformation quit curve.
- The location to the left of curve 1 corresponds to austenite (A’). The location to the proper of curve 2 represents the entire transformation of austenite (F+C). The c programming language among those curves suggests partial decomposition of austenite into ferrite and Cementite (A’+F+C).
Heat treatment process:
- Heat treating (or warmness remedy) is a collection of industrial, thermal and metalworking procedures used to regulate the physical, and every so often chemical, homes of a material. The maximum not unusualplace utility is metallurgical. Heat remedies also are used withinside the manufacture of many different materials, inclusive of glass. Heat remedy entails using heating or chilling, typically to excessive temperatures, to obtain the preferred end result inclusive of hardening or softening of a material. Heat remedy strategies consist of annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching.
- Although the time period warmness remedy applies handiest to procedures in which the heating and cooling are performed for the unique motive of changing homes intentionally, heating and cooling frequently arise by the way throughout different production procedures inclusive of warm forming or welding.
- Metallic substances encompass a microstructure of small crystals referred to as "grains" or crystallites. The nature of the grains (i.e. grain length and composition) is one of the only elements that may decide the general mechanical conduct of the metallic. Heat remedy affords an green manner to control the houses of the metallic with the aid of using controlling the fee of diffusion and the fee of cooling in the microstructure. Heat treating is regularly used to adjust the mechanical houses of a metal alloy, manipulating houses along with the hardness, strength, toughness, ductility, and elasticity.
- There are mechanisms which can alternate an alloy's houses at some stage in warmness remedy: the formation of martensite reasons the crystals to deform intrinsically, and the diffusion mechanism reasons adjustments withinside the homogeneity of the alloy. The crystal shape includes atoms which might be grouped in a completely particular arrangement, referred to as a lattice. In maximum factors, this order will rearrange itself, relying on situations like temperature and pressure.
- This rearrangement referred to as allotropy or polymorphism, may also arise numerous times, at many unique temperatures for a specific metallic. In alloys, this rearrangement may also purpose an detail to be able to now no longer generally dissolve into the bottom metallic to abruptly emerge as soluble, even as a reversal of the allotropy will make the factors both partly or absolutely insoluble. When withinside the soluble state, the procedure of diffusion reasons the atoms of the dissolved detail to unfold out, trying to shape a homogenous distribution in the crystals of the bottom metallic.
- If the alloy is cooled to an insoluble state, the atoms of the dissolved constituents (solutes) may also migrate out of the answer. This kind of diffusion, referred to as precipitation, results in nucleation, wherein the migrating atoms organization collectively on the grain-barriers. This bureaucracy a microstructure usually which includes or greater wonderful phases. For instance, metal that has been heated above the austenizing temperature (crimson to orange-hot, or round 1,500 °F (820 °C) to 1,600 °F (870 °C) relying on carbon content), after which cooled slowly, bureaucracy a laminated shape composed of alternating layers of ferrite and cementite, turning into smooth pearlite. After heating the metal to the austenite section after which quenching it in water, the microstructure can be withinside the martensitic section.
- This is because of the truth that the metal will alternate from the austenite section to the martensite section after quenching. Some pearlite or ferrite can be gift if the quench did now no longer swiftly cool off all of the metal. Unlike iron-primarily based totally alloys, maximum warmness-treatable alloys do now no longer enjoy a ferrite transformation. In those alloys, the nucleation on the grain-barriers regularly reinforces the shape of the crystal matrix. These metals harden with the aid of using precipitation. Typically a sluggish procedure, relying on temperature, that is regularly noted as "age hardening". Many metals and non-metals showcase a martensite transformation whilst cooled quickly (with outside media like oil, polymer, water, etc.). When a metallic is cooled very quickly, the insoluble atoms won't be capable of migrate out of the answer in time. This is referred to as a "diffusionless transformation."
- When the crystal matrix adjustments to its low-temperature arrangement, the atoms of the solute emerge as trapped in the lattice. The trapped atoms save you the crystal matrix from absolutely converting into its low-temperature allotrope, developing shearing stresses in the lattice.
- When a few alloys are cooled quickly, along with metal, the martensite transformation hardens the metallic, even as in others, like aluminum, the alloy will become softer.
Q9) Define austempering.
A9)
- It is very similar to martempering. Steel is austenitized and then quenched in a salt bath maintained at a constant temperature in the range of 〖260-400〗^0 C.
- The article is held at this temperature for long enough to allow isothermal transformation to be completed.
- After the complete transformation of austenite to bainite, steel is cooled to room temperature in air. It is also called isothermal quenching.
- The temperature of quenching lies below the nose of the TTT curve and above the M_s temperature.
Q10) What is martempering?
A10)
- This is a hardening method that produces martensite. This method is also known as hardening by interrupted quenching
- First the steel is heated to the hardening temperature then quenched in a medium (salt bath) having a temperature slightly above the point where martensite starts to form (usually from 〖150-300〗^0 C.
- It is held until it reaches the temperature of the medium and then cooled further to room temperature in air or oil. The holding time in quenching medium or bath should be sufficient to enable a uniform temperature to be reduced throughout the cross section but not long enough to cause austenite decomposition.
- Austenite is transformed into martensite during the subsequent period of cooling to room temperature.
Q11) Explain patenting.
A11)
- A patent is a sort of highbrow assets that offers its proprietor the criminal proper to exclude others from making, using, or promoting an invention for a restricted time frame in alternate for publishing an permitting disclosure of the invention.
- In maximum nations, patent rights fall beneathneath non-public regulation and the patent holder need to sue a person infringing the patent so that it will put in force their rights. In a few industries patents are an important shape of aggressive advantage; in others they're irrelevant.
- The technique for granting patents, necessities positioned at the patentee, and the quantity of the unique rights range broadly among nations in keeping with countrywide legal guidelines and global agreements.
- Typically, however, a patent utility need to encompass one or greater claims that outline the scope of safety this is being sought.
- A patent may also encompass many claims, every of which defines a selected assets proper. These claims need to meet numerous patentability necessities, which withinside the US encompass novelty, usefulness, and non-obviousness. Under the World Trade Organization's (WTO) TRIPS Agreement, patents have to be to be had in WTO member states for any invention, in all fields of technology, furnished they're new, contain an imaginitive step, and are able to business utility.
- Nevertheless, there are versions on what's patentable situation count from usa to usa, additionally amongst WTO member states. TRIPS additionally affords that the time period of safety to be had have to be not less than twenty years.
Q12) What is retention of austenite?
A12)
- The phenomenon of retained austenite stabilization in device steels is nicely documented. When a metallic with a martensite end temperature (Mf) under room temperature is quenched, a few austenite is retained withinside the microstructure.
- If cooling is straight away persevered to a temperature under the Mf, surely all of the austenite gift at room temperature may be converted to martensite. However, if there's a postpone among quenching to room temperature and the similarly cooling, the austenite can stabilize and can't then be converted through next cooling .
- It is critical to understand if this phenomenon additionally applies to carburized low alloy steels used to make gears. As nicely as lowering hardness and put on resistance, retained austenite withinside the case of such additives can later be converted through implemented stress, inflicting distortion in the course of service.
- Retained austenite has additionally been pronounced to result in cracking in the course of grinding after warmth treatment.
- There isn't anyt any settlement withinside the literature at the prevalence of stabilization, with a few reviews suggesting that it does now no longer arise in carburized low alloy steels . Others, however, file that it does arise and a few even endorse that its onset could be very rapid .
- Where stabilization is pronounced, it's miles normally related to excessive alloy content, mainly nickel, and with the presence of nitrogen withinside the case. The paintings pronounced right here set out to reveal the results of stabilization on a normal carburizing metallic (SAE 8620) after carburizing in a normal business cycle with none delivered nitrogen.
Q13) What is tempering?
A13)
- The tempering process provides a method for transforming martensite into ferrite and cementite. How much of the martensite is transformed depends on the temperature and time of the tempering process.
- Tempering is a heat treatment process that alters the mechanical properties (typically ductility and hardness) and relieves internal stresses of a steel. Tempering allows carbon trapped in a martensitic microstructure to disperse, and enables the internal stresses to be released from the steel that may have been created from prior operations.
The Tempering Process
- Tempering is performed by elevating the steel to a set point below its lower critical temperature, typically following a hardening operation. Once this temperature is reached, it is held there for a specified amount of time. The exact temperature and time depend on several factors such as the type of steel and desired mechanical properties.
- To get the steel to its critical temperature, some type of heating device must be used. Common devices include gas furnaces, electrical resistance furnaces, or induction furnaces. Often, this heating is done in a vacuum or with an inert gas to protect the steel from oxidation. Once the furnace achieves the desired temperature, a dwell time occurs. Following the dwell time, the furnace is shut off and the steel is allowed to cool at predetermined rate.
Why Is Steel Tempered?
- Tempering steel after a hardening process allows for a middle ground of hardness and strength. This is achieved by allowing the carbon diffusion to occur within a steel microstructure. When steel is hardened, it can become excessively brittle and hard. However, when not hardened, the steel may not have the strength or abrasion resistance needed for its intended application. Tempering also improves the machinability and formability of a hardened steel, and can reduce the risk of the steel cracking or failing due to internal stresses.
When Is Tempering Used?
- Tempering is most commonly used following a quenching operation. Heating a carbon steel and rapidly quenching it can leave it too hard and brittle. Tempering it can restore some of its ductility.
- Tempering can reduce the hardness and relieve the stress of a welded component. Welds can create a localized zone that has been hardened due to the heat of the welding process. This can leave undesirable mechanical properties and residual stress that can promote hydrogen cracking. Tempering helps prevent this.
- Work hardened materials often require tempering. Materials can become work hardened through processes such as punching, bending, forming, drilling, or rolling. Work hardened materials have a high amount of residual stresses that can be alleviated through a tempering process
Q14) Explain flame and Induction hardening.
A14)
- Induction hardening includes the use of brought about electric currents to very unexpectedly generate warmth through hysteresis, typically in a workpiece crafted from medium to excessive carbon steel.
- Flame hardening makes use of oxy-gasoline burners to warmth the workpiece through conduction. Both methods use quenching after heating, regularly observed through tempering and/or pressure relieving.
- Induction hardening Surface of thing heated via way of means of excessive frequency induction, the frequency and energy necessities relying on length and geometry of thing and intensity of hardening required.
- Three essential kinds of induction machines: vacuum tube oscillator > 500 kHz spark hole oscillator 10–30 kHz motor generator 5–10 kHz Heat-up instances may be managed to within ±0.1 s and have to be much less than 20 s to save you distortion and keep away from post-remedy machining or grinding. Process also can be used for via hardening of small components. Tempering and/or pressure relieving also can be performed via way of means of induction heating, regularly with the identical induction coil.
- Process is without difficulty automatic and may deliver uniform high-satisfactory and repeatability, even though excessive capital and going for walks charges make it maximum appropriate for lengthy manufacturing runs. Typical packages encompass gears, crankshafts and camshafts.
- Materials: Ferrous metals whose composition is such that the specified hardness might be acquired on quenching from the austenite region: at the least 0.3% C. Most substances have as a minimum 0.4% C, and as much as 0.7% C. Plain carbon steels, alloy steels and grey, nodular and malleable solid irons; the temperature relies upon at the material: 750–760° C 0.5% undeniable carbon 900–800° C chromium–molybdenum alloy steels 950–980°C nodular iron Alloy steels, despite the fact that extra expensive, produce extra case depths.
- The better the carbon content, the extra the threat of cracks and/or distortion. Flame hardening isn't always generally appropriate for steels with middle strengths of extra than 900 MPa. Required middle electricity need to be acquired through suitable warmness remedy earlier than hardening.
- To permit carbides to enter answer at some stage in the fast heating times, the carbides must be small, for this reason normalised systems are preferred. Fine grains deliver higher manage over the case intensity and much less hazard of cracking and/or distortion. Very little grain grown occurs, because of quick heating times.
- Little or no decarburising or oxidation because of quick heating times.
- Surface hardnesses depend upon composition, temperature and quenchant, however are generally withinside the variety 350–seven-hundred Hv.
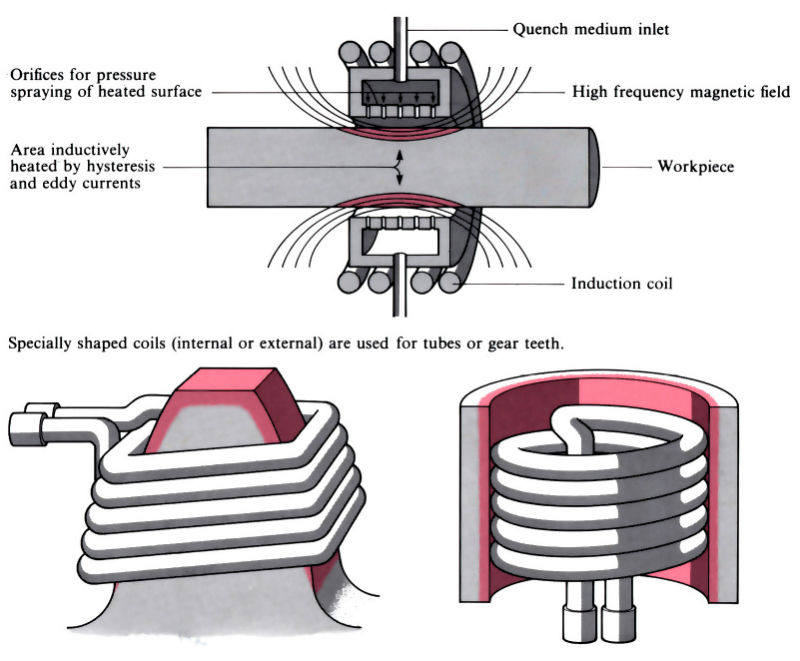
Fig: Flame and Induction hardening