Unit – 1
General Geology, Mineralogy and Petrology
Q1. Describe the importance of Geology?
A1.Geology is the study of the earth (geo -means earth, and ology- means study of).Geologists study some of society most important problems, such as energy, water, and mineral resources; the environment; climate change; and natural hazards like landslides, volcanoes, earthquakes, and floods.
Further geology also includes the study of various physical, dynamic and physiochemical process operating on or within the earth and of the agents and force involved and evolved in such process. Geology is rightly considered as one of the fundamental basic sciences like physics, chemistry and biology.
Q2. Describe the scope of Geology studies?
A2 .Scope of Geology:-
1. Knowledge of ground water Geology is necessary in connection with excavation works, water supply, irrigation and other purposes.
2. Wetland and habitat restoration programs.
3. It investigate geological phenomena such as earthquakes and volcanoes.
4. Geology is useful to know the method of mining of rocks and mineral deposit on earth surface and subsurface
5. For coastal engineering, sand replenishment, bluff or esa cliff stabililty, harbour, pier etc.
6. For planning, Designing, and construction of civil related works.
7. For governmental and military installation.
Q3. Define silicate and non-silicate mineral?
A3. Silicate & Non silicate minerals
Silicate Minerals
Composition of Silicates
Non-Silicates
Q4. Explain the various physical properties of mineral with types and example?
A4. The various physical properties of minerals are as follows:-
1. Colour
2. Luster
3. Streak
4. Hardness
5. Cleavage
6. Parting
7. Fracture
8. Tenacity
9. Structure
10. Form
11. Specific Gravity
Classification of Minerals
A mineral may belong to any one of three types:-
A] Idiochromatic-fairly constant colour related primarily to the composition of minerals.
Example are as follows malachite, peridot, and almandine.
B] Allochromate-variable colour, examples are as follows like quartz, calcite, Fluorite etc. Basically this colour impart due to impurity.
C] Pseudochromatic- Thos colour are due to light diffraction and the colour is false colour.
2. Luster:-it is called as shine of mineral or reflection of light from surface of minerals. It is important to know that whether a mineral is metallic or not for the properties of luster, which are given as:-
1. Metallic -looks like a piece of broken or polished metal. Examples are pyrite and galena.
2. Submetallic - has a high luster that is transitional between that of broken metal and that of broken glass. An example is black sphalerite.
3. Nonmetallic-does not look like metal.
Properties of luster:-
A] Reflective surfaces
B] Capacity of mineral to absorb light
C] Refractive index (Since refractive index is a fundamental physical property of a substance, it is often used to identify a particular substance, confirm its purity, or measure its concentration.)
3. Streak:-The colour of a mineral when it is powdered is called the streak of the mineral. Crushing and powdering a mineral eliminates some of the effects of impurities and structural flaws, and is therefore more diagnostic for some minerals than their colour.
4. Hardness:-The resistance of mineral which offer outer changes example as scratching, abrasion, rubbing etc. are called as Hardness of minerals. OR The hardness of a mineral is its ability to resist scratching, the mineral hardness scale of Mohs is based on the ability of one natural mineral sample to visibly scratch another mineral. All different minerals are the samples of matter used by Mohs. Minerals are naturally found pure substances. Rocks consist of one or more minerals. Diamonds are at the top of the scale as the hardest known naturally occurring substance when designing the scale
The mohs scale of hardness comprises ten mineral arranged in order of ascending hardness, the softest is assigned a value of 1 and hardest as 10.
Mohs hardness | Mineral | Chemical formula |
|
|
1 | Talc | Mg3Si4O10(OH)2 |
|
|
2 | Gypsum | CaSO4·2H2O |
|
|
3 | Calcite | CaCO3 |
|
|
4 | Fluorite | CaF2 |
|
|
5 | Apatite | Ca5(PO4)3(OH−,Cl−,F−) |
|
|
6 | Orthoclase Feldspar | KAlSi3O8 |
|
|
7 | Quartz | SiO2 |
|
|
8 | Topaz | Al2SiO4(OH−,F−)2 |
|
|
9 | Corundum | Al2O3 |
|
|
10 | Diamond | C |
|
|
5. Cleavage:-It is defined as the mineral to break along certain definite direction yielding more or less smooth, plane surfaces. Cleavage is the result of weaker bond strengths or greater lattice spacing across the plane in question than in other directions within the crystal. Greater lattice spacing tends to accompany weaker bond strength across a plane, because such bonds are unable to maintain a close interatomic spacing
6. Fractures :- Fracture is the tendency of a mineral to break along curved surfaces without a definite shape. These minerals do not have planes of weakness and break irregularly.
Common type of fractures are as follows:-
1] Even: example Chert
2] Uneven: ex Fluorite
3] Conchoidal: ex Quartz
4] Hackly: ex Native copper
5] Earthy: ex Chalk
6] Splintry: ex Kyanite.
Q5. Define hardness of mineral. Also define Mohs scale of hardness?
A5. Hardness:-The resistance of mineral which offer outer changes example as scratching, abrasion, rubbing etc. are called as Hardness of minerals. OR The hardness of a mineral is its ability to resist scratching, the mineral hardness scale of Mohs is based on the ability of one natural mineral sample to visibly scratch another mineral. All different minerals are the samples of matter used by Mohs. Minerals are naturally found pure substances. Rocks consist of one or more minerals. Diamonds are at the top of the scale as the hardest known naturally occurring substance when designing the scale
Mohs hardness | Mineral | Chemical formula |
|
|
1 | Talc | Mg3Si4O10(OH)2 |
|
|
2 | Gypsum | CaSO4·2H2O |
|
|
3 | Calcite | CaCO3 |
|
|
4 | Fluorite | CaF2 |
|
|
5 | Apatite | Ca5(PO4)3(OH−,Cl−,F−) |
|
|
6 | Orthoclase Feldspar | KAlSi3O8 |
|
|
7 | Quartz | SiO2 |
|
|
8 | Topaz | Al2SiO4(OH−,F−)2 |
|
|
9 | Corundum | Al2O3 |
|
|
10 | Diamond | C |
|
|
Q6 .explain rock forming mineral?
A6. Feldspars
Feldspars (KAlSi3O8–NaAlSi3O8–CaAl2Si2O8) is a collection of rock-forming tectosilicate minerals which make up by weight about 41% of the mainland surface of the Earth. In both intrusive and extrusive igneous rocks, feldspars crystallize from magma as veins and also available in many kinds of metamorphic rock. It is known as anorthosite rock made almost of calcium plagioclase feldspar.
Quartz
It is a mineral composed of carbon and water particles in a constant frame of SiO4 silicon-oxygen tetrahedra, share each carbon between two tetrahedra, giving SiO2 a general chemical formula. Quartz is Earth’s second most common mineral, behind feldspar, in the continental crust.
Two forms of quartz, the normal α-quartz and the β-quartz high-temperature, both chiral. There is a huge transformation from α-quartz to β-quartz at 573 ° C (846 K). Since the transition is followed by a quantity shift, ceramics or rocks that pass through this temperature limit can readily be induced to fracture.
Amphibole
Amphibole is a significant cluster of inosilicate minerals which form prisms or needle-like crystals, made up of SiO 4 tetrahedra double chain, connected at the vertices and usually carrying ions of iron and/or magnesium in their constructions. It can be green, black, white, yellow, blue, or brown. It is presently classified by the International Mineralogical Association as a mineral super group, in which there are two categories and several subgroups.
Mica
Mica group of sheet silicate (phyllosilicate) minerals consist of several near-perfect basal cleavage associated products. They are all monoclinic, with a tendency to pseudo hexagonal crystals, and in chemical composition are close.
The term mica comes from the Latin term mica, it means a crumb to glitter, and is likely affected by micare.
Olivine
Mineral olivine is a formula (Mg2 +, Fe2+) 2SiO4 zinc iron silicate. It is a kind of nesosilicate or orthosilicate.
Olivine consist of only small quantities of non-oxygen, silicon, magnesium and iron components. The extra components found in the greatest levels are manganese and nickel.
Garnet
Garnets are a set of minerals of silicate which is used as gemstones and abrasives since the Bronze Age.
All garnet species have special physical and crystal shapes, but vary in chemical composition. The various species are pyrope, almandine, spessartine, gross, uvarovite and andradite.
Two solid solution series are made up of garnets: pyrope-almandine-spessartine and uvarovite-grossular-andradite.
Calcite is a mineral carbonate and stable calcium oil polymorph (CaCO3). The mineral hardness scale of Mohs, based on the contrast of scratch hardness, describes value as “calcite”.
Pyroxenes
Pyroxenes (frequently shortened to Px) are a set of significant minerals found in many igneous and metamorphic rocks which form rock ino-silicates. Pyroxenes have overall formula XY (Si, Al)2O6 where X depicts calcium, sodium, iron (II) or potassium and commonly zinc, manganese or lithium, and Y includes ions of lower magnitude such as chromium, aluminium, iron (III), magnesium, cobalt, manganese, scandium, titanium, vanadium or even metal (II).
In silicates such as feldspars and amphiboles, aluminium widely replaces silicon, the replacement happens in most pyroxenes only to extent. It share a framework made up of silica tetrahedra single chains.
Q7. Explain igneous rock with different texture?
A7. Textures defined the size, shape and arrangement of these constituents within the body of rocks. The grain size of an igneous rocks depends on the rate of cooling of magma, for this 4 points are considered:-
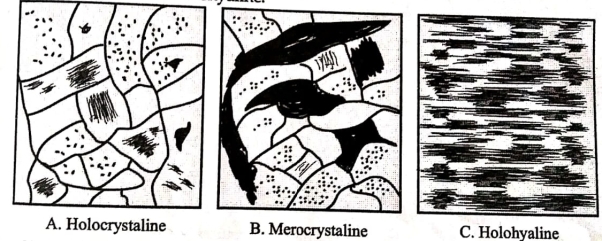
1. Degree of crystallization:-
A] Holocrystalline Texture:-Rock is made up of full crystal is defined as Holocrystalline
B] Merocrystalline texture:-Rock is made up partly by glass and partly by crystal is defined as merocrsytalline.
C] Holohayaline texture:-Rock is made up of full glassy material is defined as Holohayaline.
2. Size of grains:-
In rapid cooling the mineral grains crystallize quickly as a mass of tiny crystals which are generally less than 1 mm in size. Some common grain shapes are:
The texture of phaneric igneous rocks can be further subdivide into as:-
1. Coarse grained (more than 5mm in diameter)
2. Medium Grained (1mm to 5mm in diameter)
3. Fine grained (less than 1mm in diameter)
4. Microcrystalline (can be seen by microscope)
5. Cryptocrystalline (texture are very small i.e. not visible)
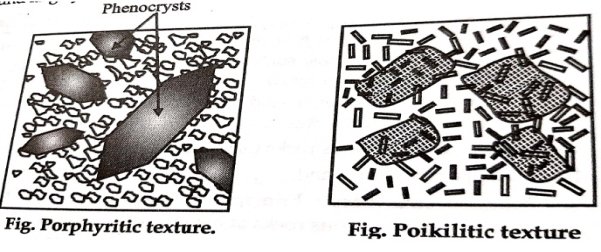
3. Shape of Crystal:-
They are as follows:-
1. Euhedral (crystal faces)
2. Subhedral (partly crystal faces)
3. Anhedral (crystal faces are absent)
4. Mutual Relations of Grains:-
These are classified as four major groups which are as follows:-
A] Equigranular texture
B] InEquigranular texture
C] Directive texture
D] Intergrwoth Texture
Q8. Define sedimentary structure?
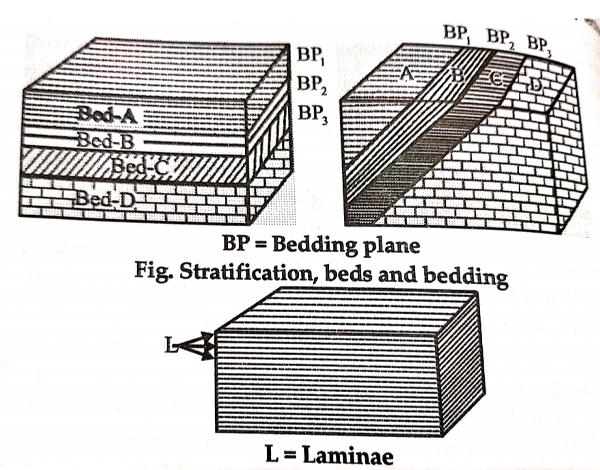
A8. Sedimentary structure:-
.Planes of weakness –the bedding planes separate the bed from each other. Large scale cross-bedding in sandstone, within horizontal layers a few to many feet thick, indicates deposition in sand dunes.
2. Ripple marks-these are commonly seen in shallow water environment. They are defined by asymmetric and symmetric ripples. Ripple marks indicate deposition in a current. Asymmetric ripples (one side steeper than the other) indicates a consistent current direction as in streams. Symmetric ripples indicate oscillating (waves) or weak currents.
3. Mud cracks-these are the common structural features of many fine grained sedimentary rocks. Mud cracks are produced by drying of wet muds. Once these cracks are covered under further layer of mud, they get preserved in the body of deposit. Raindrop impression may also be preserved in sediments. They indicate deposition in a terrestrial setting.
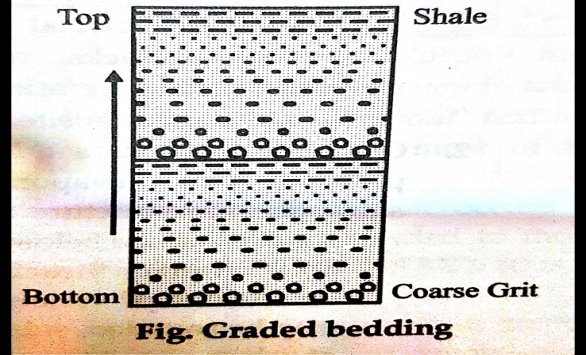
4. Fossiliferrous structure is are very important indicators of depositional environment. Sedimentary rocks are known as the only source of fossils. Fossils include preserved skeletal fragments, plant roots, etc., and also trace fossils such as burrows, footprints, leaf impressions, etc. Coral and many shell fossils indicate marine deposition.
5. Rain prints- It is also like mud cracks. They are formed irregular small crater shaped depression seen on fine grained dried sediments.
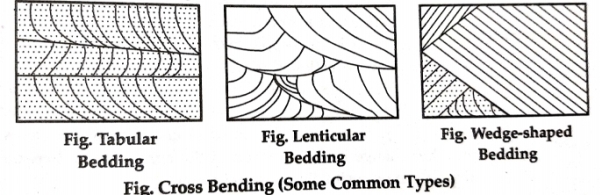
6. Cross Bedding-Internal stratification within a larger bed may be parallel or there may be cross-stratification caused by ripples, sand bars, and dune structures.
7. Lamination-layered structure similar to bedding as found in sedimentary rocks. The individual layer are called as lamina and are distinguished commonly on the basis of difference in colour.
Q9. Describe Lithification?
A9.
The common method of lithifying coarse grained sediments is by CEMENTATION. Sedimentary particles are deposited in touch with each other, but there is also a certain amount of void space in a pile of sediment. As water carrying dissolved ions fills in that empty space, the ions may crystallize new minerals between the grains
Q10. Enumerate the various types of Metamorphism?
A10. The various types of metamorphism are as follows:-
1. Cataclastic metamorphism
2. Dynamic metamorphism
3. Contact metamorphism
4. Plutonic metamorphism
5. Regional metamorphism
6. Metasomatism and
7. Retrogressive metamorphism.
1. Cataclastic metamorphism:-
When directed pressure or lateral stress play dominant role in metamorphism then the process is called as Cataclastic metamorphism. Cataclastic metamorphism occurs as a result of mechanical deformation, like when two bodies of rock slide past one another along a fault zone. Heat is generated by the friction of sliding along such a shear zone, and the rocks tend to be mechanically deformed, being crushed and pulverized, due to the shearing. Examples are mylonites, etc.
3. Dynamic metamorphism:-
When there is some increase in temp. With high pressure then process is called as dynamic metamorphism. These ultrahigh pressures can produce minerals that are only stable at very high pressure, such as the SiO2 polymorphs coesite and stishovite. In addition they can produce textures known as shock lamellae in mineral grains, and such textures as shatter cones in the impacted rock.
4. Contact metamorphism:-
The other name is given as THERMAL METAMORPHISM. Contact metamorphism occurs adjacent to igneous intrusions and results from high temperatures associated with the igneous intrusion.
Since only a small area surrounding the intrusion is heated by the magma, metamorphism is restricted to the zone surrounding the intrusion, called a metamorphic or contact aureole. Outside of the contact aureole, the rocks are not affected by the intrusive event.
5. Plutonic metamorphism:-
In the high depth of earth high temp. And static load works together the metamorphism caused by these factors is called the plutonic metamorphism. Examples are Granulites.
6. Regional metamorphism:-
When pressure and heat act together in the hydrothermal fluids the rocks are metamorphosed over the wider area this is the logic behind the Regional Metamorphism. Regional metamorphism occurs when rocks are buried deep in the crust. This is commonly associated with convergent plate boundaries and the formation of mountain ranges. Because burial to 10 km to 20 km is required, the areas affected tend to be large. Examples are foliate rocks splits easily into flaky sheets.
6. Retrogressive metamorphism:-
When high temperature metamorphic mineral assemblages are change to low temperature mineral assemblages the process is called as Retrogressive metamorphism. In general, the changes in mineral assemblage and mineral composition that occur during burial and heating are referred to as pro grade metamorphism, whereas those that occur during uplift and cooling of a rock represent retrograde metamorphism