Unit - 3
Design Of Water Treatment Plant
Q1) Explain what do you mean by coagulation and flocculation? Explain necessity of coagulation.
A1)
- The word has been derived from a Latin word 'flocculator', it means, to form a floc.
Flocculation is stimulation by mechanical methods to collect destabilized particles into compact flocs.
- When these particles come in contact, they become large and get settled in the form of flocs.
- The flocculation is the result of changes in the velocity of the water which is mixed with coagulant.
- Coagulation means to add the chemicals, called as coagulant, which react with the impurities and convert them it to sizable and settleable volumes.
Necessity of coagulation:
- Generally, in a case of the public water supply project; the source of water is the surface water.
- This water as it remains open, has a lot of impurities in the suspended form making it turbid.
- Sometimes the colour of the water also is changed due to colloidal matter and the dissolved organic material. So, it has a turbidity due to clay, silt and some organic matter.
- Unless, such water is allowed to settle down for a longer period in the sedimentation tank, these impurities are not removed, or it can be done by making the size of the suspended water larger and bigger; to allow it to settle down.
- Coagulation means to add the chemicals, called as coagulant, which react with the impurities and convert them it to sizable and settleable volumes.
- This method of coagulation is used when the turbidity of water is more than 40 p.p.m. (parts per million).
- One must know that coagulation is not, complete method of water purification. It helps to remove the impurities, when used in the plain sedimentation. It is necessary to follow the methods of filtration for the final removal of the impurities to make it safe and portable.
Q2) Explain principles of coagulation in detail.
A2)
- The base of the process of coagulation is that the stability of colloidal particles depends upon the electric charge which they have or they possess.
- The primary charge of the colloidal particle is due to the charged groups, which are within the surface of the particles or may be due to the process of adsorption of the layer of ions, from the surrounding medium.
- This primary charge gets counter balanced by the ions of opposite charge in the water phase.
- Between the water and the solid an electrical double layer is formed at the interface.
- The double layer has,
(i) The charged colloidal Particles.
(ii) The equivalent excess of oppositely charged counter ions they get accumulate in the water, near the surface of the particles.
- The counter ions are attracted electro-statically to the particle surface and they cause the high concentration at the particle surface.
- This diffusion or decrease of these counter ions, is due to the agitation and also due to replacement by other ions.
- When there is a high concentration of counter ions, in the water the diffused layer is compacted. It is known as the diffused double layer theory.
- Common coagulants alum and ferric salt:
Alum:
It is also called as 'Aluminium sulphate'. Its use in the water treatment plant is almost worldwide. It is supply in the form of flakes or in the form of solid lumps and while applying for the treatment, it is converted into a solution.
a) Advantages of Alum
- It helps to reduce the bad odour, taste and also reduces the turbidity of the water.
- The price of Alum is low.
- The use of Alum does not require skill full workers to handle so the cost goes still low.
- Alum, after its reaction, creates almost crystal clear water.
- The floc formed by Alum, is very tough and does not break easily.
- The dosage of Alum depends upon the turbidity of water, pH value, temperature of water, colour and taste of water etc. The range of Alum requirement for the treatment varies between 5 and 30 milligrams per lit.
- For the normal conditions of water, the dosage is about 14 milligrams per lit.
b) Disadvantages of Alum
- The sludge developed after the use of Alum is difficult to dispose off. It is also has been observed that this sludge cannot be used for filling the low-lying areas.
- To maintain the pH value, together with alum, caustic soda or the lime is required to be added.
- This increases the cost of the process.
Ferric Salts:
- The effective PH range is 8.50 and above.
- If floc is heavier than the floc formed by the alum.
Q3) What is bentonite clay?
A3)
Bentonite clay:
- The goal of this paintings is to look at the effectiveness of bentonite as a coagulation adjuvant on water remedy excellent at Ait Baha station. In our case, bentonite is used as a supply of turbidity and isn't an adsorbent.
- Jar exams have been done on uncooked water samples of low turbidity in keeping with the technique implemented withinside the laboratory. The overall performance of the bentonite became examined in mixtures: uncooked water/bentonite, uncooked water/coagulant and coagulant/bentonite.
- The most important targets of this have a look at are to confirm whether or not bentonite acts as a coagulation resource for water remedy, after which to limit the dose of aluminum sulfate at the same time as respecting the remedy yield of the station. Indeed, the outcomes received confirmed that the addition of bentonite doses of 20 mg/L can put off 96.72% of the turbidity and 60% of the oxidizable material.
- Also, the boom of the dose of bentonite decreases the pH extra than using aluminum sulfate alone, which makes the water pH ultimate for coagulation–flocculation, however additionally to enhance the coagulation and flocculation strategies to attain an awesome excellent effluent and the speedy sedimentation of the flocs formed.
Q4) Explain limestone in detail.
A4)
Lime stone:
- Lime is utilized by many municipalities to enhance water quality, mainly for water softening and arsenic removal. Indeed, the American Water Works Association has issued requirements that offer for the usage of lime in ingesting water remedy. Softening - In water softening, hydrated lime is used to eliminate carbonate "hardness" from the water.
- Hardness as a result of different calcium and magnesium salts, referred to as noncarbonate hardness, is typically dealt with via the lime-soda process, which includes the precipitation of magnesium by means of lime.
- The co-produced calcium salt reacts with the soda ash to shape a calcium-carbonate precipitate. Lime-better softening also can be used to eliminate arsenic from water.
- Stricter ingesting water guidelines for arsenic have improved the want for this remedy. PH Adjustment/Coagulation - Hydrated lime is extensively used to modify the pH of water to put together it for similarly remedy. Lime is likewise used to combat "pink water" by means of neutralizing the acid water, thereby lowering corrosion of pipes and mains from acid waters.
- The corrosive waters incorporate immoderate quantities of carbon dioxide. Lime precipitates the CO2 to shape calcium carbonate, which gives a protecting coating at the inner of water mains. Lime is used alongside alum or iron salts for coagulating suspended solids with a view to eliminate turbidity from water. It serves to keep the right pH for maximum great coagulation conditions.
- In a few water-remedy plants, alum sludge is dealt with lime to facilitate sludge thickening on strain filters. Effect on Pathogen Growth - By elevating the pH of water to 10.5-eleven thru the addition of lime and maintaining the water in touch with lime for 24-seventy-two hours, lime controls the surroundings required for the increase of micro-organism and positive viruses.
- This software of lime is applied where "phenolic water" exists, due to the fact chlorine remedy has a tendency to supply unpalatable water because of the presence of phenol. This process, referred to as 'extra alkalinity remedy', additionally eliminates maximum heavy metals. Removal of Impurities - One of the maximum not unusual place techniques of getting rid of silica from water includes the usage of dolomitic lime.
- The magnesium factor of this lime is the lively constituent in silica removal. Lime is likewise used to eliminate manganese, fluoride, natural tannins and iron from water supplies.
Q5) What do you mean by silicate?
A5)
Silicate:
- Four experiments of coagulation and flocculation had been performed to analyze the traits of colloidal silica elimination in a high-tech commercial wastewater remedy plant for reclamation and reuse of the effluent. Experimental consequences illustrated that poly-aluminium chloride (PACl) confirmed better performances on colloidal silica elimination than alum.
- Interestingly, the 2 coagulants tested the identical capability on silica elimination. The unique silica elimination capability changed into about 0.one hundred thirty-five mg SiO2/mg Al2O3 while the dosage of coagulants changed into withinside the variety 30-one hundred fifty mg/L Al2O3. In addition, the silica changed into decreased substantially on the circumstance of pH above 8.
- Experimental information implied that precipitation of aluminium flocs changed into the predominant mechanism for colloid silica elimination in PACl and alum coagulation, besides, fee adsorption changed into additionally essential for enhancing elimination efficiency.
- Moreover, the addition of polyacrylic acid (PAA) as a flocculant may want to barely develop silica elimination withinside the PACl coagulation. The blended PACl/PAA/flocs coagulation changed into powerful for the elimination of colloidal silica, soluble COD, and turbidity and additionally appropriate as a pretreatment unit in wastewater reclamation and reuse processes.
Q6) Explain polyelectrolytes.
A6)
Polyelectrtolytes:
- They are the polymers and on the basis of the charge they carry, they can be classified as anionic, cationic, and non-ionic. Among these only cationic polyelectrolytes can be used very effectively as the independent coagulant. While in cases of other varieties, they can be used together with Alum or any other conventional coagulants.
- In India polyelectrolytes are collected from the extract of Nirmali seeds. This type of deciduous plant, small in size is found in almost all the state in India but mainly in Andhra Pradesh, Konkan coastal region of Maharashtra State, Madhya Pradesh, Orissa and in West Bengal.
- The use of polyelectrolytes needs to have a skilled supervision because if the dose is more than required, it may affect the working of filters.
- We must note here that the use of these polyelectrolytes as the coagulants is still in the pilot stage and so they may prove to be a better alternative for other coagulants in future.
Q7) What do you mean by natural coagulation? Explain concept of mean velocity.
A7)
Introduction to natural coagulants:
- In the past, the natural coagulants of vegetable and of marine origin, were used for the water and also for wasted water treatment, before the synthetic chemical such as Alum and ferric salts, were introduced. Without any scientific reasoning the natural coagulants were discouraged.
- Now, a number of effective natural coagulants have been identified from the plants. For example, Moringa, Nirmali, Okra, Apricot, Beans, Rice, Maize etc. This use of natural coagulant has helped to reduce the cost of water treatment.
- Moringa Oleifera (M.O.) is widely used in most of the developing countries. It has the following advantages:
- It has good coagulation property without the constrains of alum.
- The sludge produced by M.O. Is very compact (about 4 to 5 times, compact, then produced by alum).
- The efficiency to remove turbidity is about 95%.
- M.O. Is cheaper coagulant.
- The velocity gradient of two fluid particles which are 0.05 feet apart, with relative velocity of 0.2 fps is equal to, 2fps / 0.05 ft = 40fps / f t
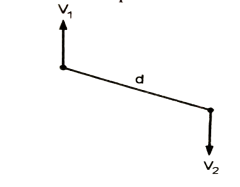
Fig: Mean Velocity
6. The rate of particle collision α G
7. Shear force α 4
8. Total number of particle collision α GT
9. The power consumption in the process depends upon the speed of mixing the amount coagulants to be mixed and the volume of water.
Q8) Explain design of flocculation chamber.
A8)
- The word has been derived from a Latin word 'flocculator', it means, to form a floc.
- Flocculation is stimulation by mechanical methods to collect destabilized particles into compact flocs.
- When these particles come in contact, they become large and get settled in the form of flocs.
- The flocculation is the result of changes in the velocity of the water which is mixed with coagulant.
- This process takes place in the mixing basin also known as flocculation basin.
- It is common practice to provide the flash or rapid mix which is followed by the slow mixing.
Design of flocculator:
- See the Fig. which shows the design of a typical flocculator. In this the slow mixing is achieved by rotating the paddles with two to three revolutions per minute.
- Sometimes the flocculator is combined with the sedimentation tank. This type of tank is known as coagulation sedimentation tank.
- A floc chamber is attached before the water enters in the sedimentation tank the period of detention in the floc chamber is about 15 to 40 minutes and detention period for the tank is about 3 to 4 hours. The cleaning of the tank is carried out after 3 to 6 months.
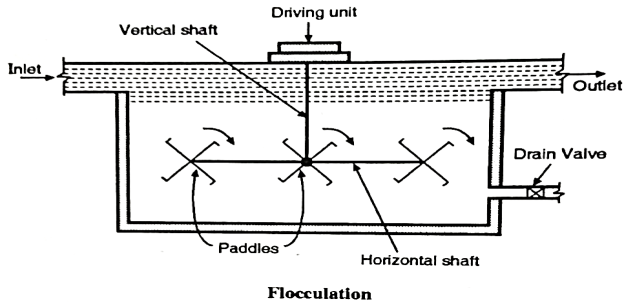
Fig: Flocculator
Q9) Explain various terms used in design of flocculation chamber.
A9)
Important terms- Design of flocculator:
- Detention period
"It is the period of time given to settle the row water to settle down the impurities".
2. Discrete particles
"It is size shape of the particles which varies between 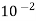 and
and 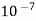 cm”
cm”
3. Surface loading
"It is process of mixing the chemicals for the precipitation of impurities".
4. Mean velocity gradient
"It is the rate of change of velocity per unit distance normal to a section i.e., meter sec. Meter". So, G (Gradient) is expressed as sec
5. Weir loading
If the water falls over a weir, through same out let it is called as weir loading. It is expressed as "The quantity of water discharged per day or per hour, per unit length of the weir.
6. Flocculation
It is the result of changes in the velocity of the water which is mixed with coagulant.
7. Surface overflow rate
"Surface overflow or surface loading is the quantity of water i.e., volume of water passing per day or per hour, per unit horizontal area."
Q10) Explain design criteria for flocculator provided with rotating paddle.
A10)
Following are the various design criteria commonly involved in the design of flocculator:
- Depth of tank is taken from 3m to 4.5 m.
2. Detention time is considered as 10 minutes to 40 minutes and normal detention time is taken as 30 minutes.
3. Length to width ratio is considered as 2 to 3 for rectangular type of basin.
4. Mean velocity gradient (G),
G =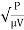
Where, G = Mean velocity gradient (sec¹)
P = Power dissipated in 'watt'.
μ = Absolute viscosity (N-s/m²)
V = Volume to which power is applied (m³).
- The flocculation technique most commonly used mechanical agitation with rotating paddle wheels or vertically mounted turbines:
P = Power input by the impeller
= 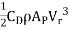
Where 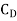 = Coefficient of drug of paddle
= Coefficient of drug of paddle
= 1.8 for paddle with flat paddles
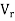 = Relative velocity of impeller and fluid
= Relative velocity of impeller and fluid
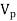 = Velocity of the tip of the paddles
= Velocity of the tip of the paddles
V = Velocity of water adjacent to the tip of the paddle
- The optimum value of G can be determined
Q11) Explain design of flocculator and clariflocculator.
A11)
Flocculator design
- Detention time, discharge (Q) in m'/min are considered.
- Volume of flocculator chamber = Q x detention time
- Assume depth of flocculation zone.
- Surface area of tank
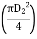 =
= 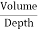
- Find D₂ i.e., diameter of flocculator provide control chamber for flocculator of diameter 'D₂'
Clariflocculator design
1. For given discharge Q in m3/min and detention time find volume
Volume = Qx detention time
2. For a given surface loading, find the area of tank.
Area of tank = 
3. Find clarifier surface area (in ring form)
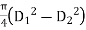 = Area of tank
= Area of tank
4. Find depth
Depth =
5. Find the overall depth by providing additional depth for sludge and for free board.
Q12) Explain theory and mechanism of filtration.
A12)
- Even after the process of sedimentation and the process of coagulation which removes the impurities present in the water, the water still is not fully pure for consumption as it may still have some very micro particles or some fine micro-organic matter.
- To remove these fine particles from the water, the water is allowed to pass through the beds of granular material. This process is known as filtration.
- After this process of filtration, the water becomes free from all the undesirable impurities such as colour, odour, turbidity and also the pathogenic bacteria.
Mechanism of filtration:
- In the process of purification of water, the filtration is the most important stage. The water is allowed to pass through a thick layer of sand to remove all the remaining impurities of water to make it portable and to make it safe for drinking purpose. This process brings following changes in the water, which is getting treated.
- The suspended and colloidal impurities are removed from the water, to a great extent.
- The chemical characteristics of the water get changed.
- The proportion of bacteria in the water is reduced.
- These changes are the results of the four actions which take place during the process of filtration. They are as follows:
Filtration Process
- Mechanical straining
- Sedimentation
- Biological metabolism
- Electrolytic changes
Let us study the functions of these actions in process of filtration.
- Mechanical straining
- The suspended particles which cannot pass through the sand, are trapped and are removed by this action.
2. Sedimentation
- The void between the sand particles acts as a small size sedimentation tank and the impurities are arrested either due to coating developed on the sand particles by the colloidal matter saturated on the sand or by the physical attraction between two particles of any matter.
3. Biological metabolism
- The life process of any living cell is called as biological metabolism. When any type of bacteria is caught in the voids of the sand particles a thin jell or a film is developed around the particles of sand having a very large number of bacteria which use the organic impurities as a food and convert them into harmless compounds to make the water safe for drinking.
4. Electrolytic changes
- When any two deflect particles have opposite electric charges are put together, the electrical charges are totally neutralized to develop a new chemical substance which is not harmful. They are needed to be removed by cleaning the filters to make it safe and portable.
Q13) Explain filter material in detail.
A13)
- Base materials:
- The base material of gravel is placed on the top of the under-drainage system. The depth of the gravel material varies between 300 mm and 750 mm. The size of the gravel’s changes from the top to the bottom layer e.g., the top layer has the gravels having the size between 3mm and 6mm.
- In the intermediate layer, the size varies between 6 mm and 20 mm (in some cases it may have the size of the gravel between 20 mm and 40 mm). While in the lowest i.e., bottom layer.
- The size of gravel varies between 40 mm and 65 mm.
Out of the total depth of the base material of gravel the top layer is about 150 mm, in depth, the middle or the intermediate layers.
- Cover 150+ 150 mm deep and bottom layer also is 150 mm deep. So, the total depth of these layers is 600 mm.
2. Filter media of sand:
- Above the layers of grave a layer of sand is placed.
- The depth of this sand layer varies between 600 mm and 900 mm and size of the sand particles also varies from 0.20 mm to 0.30 mm.
- Fine sand can be more effective to remove the impurities but the time required for the process is long and processes is slow.
Q14) What is rapid sand filter?
A14)
Rapid sand filter:
- Though the slow sand filter has a lot of advantages and good for the rural water treatment plant, the greatest disadvantage of the slow sand filter is that it needs to have a large space of its installation (in the rural areas, space is not a major problem).
- So, in the urban areas where the land cost is sky hill, that method becomes useless e.g., for a moderately populated town say about 15000, the area required for the installation of the slow sand filter plant is about 1000 m², and considering the future water demand and the space required for other attached equipment for water purification, about 2000 m² would be necessary.
- So, it would be too costly to follow the slow sand filtration method. So, the rapid sand filtration method has been put to use.
- Methods of Rapid Filtration
- The rate of filtration can be increased by using two different methods, such as
It can be achieved as increasing the size of the sand particles. This reduces the friction of sand particles with the moving water. This helps to gain high speed for the filtration.
- If the water allowed to pass through the sand, with pressure, its velocity increases and so the time required for filtration is reduced.
B. Design of the Rapid Sand Filters
- As shown in the Fig. It has five essential parts such as enclosure tank, under drainage system, base material, filter media of sand and appurtenances. Let us study the functioning of these parts.
Q15) Explain perforated pipe system and pipe and strain system.
A15)
Perforated pipe system
- In this system, various lateral drains are attached to the central drain. These drains are made up of cast-iron. They are placed with the average distance between 150 mm and 300 mm. The pipes of the drains have holes with 10 mm diameter and having the angle of 30° between two holes. To control the rusting of the holes, brass bushings are used to protect the holes.
- This system of perforated pipe is very simple and need less cost also but it requires about 700 lits. Of water per minute - per m² of the filter area for the washing purpose.
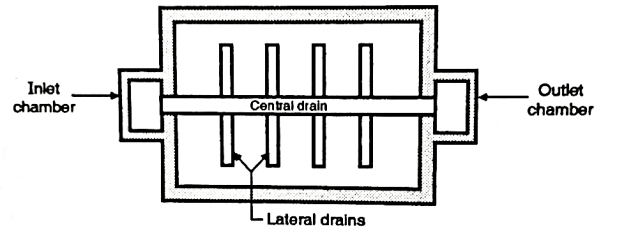
Fig: Perforated pipe system
Pipe and strainer system
- In this system also, there is a central drain system but instead of wholes, this system has strainers. It is a small pipe made of brass. It has holes on its surface but it is closed at the top. They are fixed on the top of the drain. A compressed air is used for washing the filters. It needs about 250 litres of water per minute per m² of the filter area, for washing. It is called as low velocity wash.
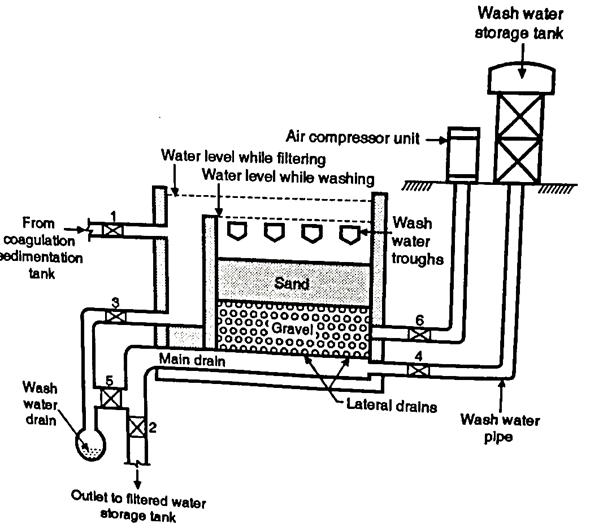
Fig: Pipe and strainer system