Unit 01
Dielectric properties of insulating materials
Q1) What properties have insulating materials?
A1) Properties of the insulating materials are listed below and described.
Electrical property
Thermal property
Chemical property
Mechanical property
- Electrical property of the insulating materials
a) Insulation resistance
Resistance between two conductors are separated by usually insulating materials. Between two parallel paths it is the total resistance, one is through the body and another over the surface of body.
b) Resistivity
This is measured by the insulation resistance. When this term is applied to the insulating materials requires qualifications to volume or surface.
Volume resistivity
This is the resistance between opposite faces of the cube of the unit dimensions, it is generally explained in the mega ohm-centimeters.
Surface resistivity
This is the resistance between opposite sides of the square of the unit dimensions o surface materials, it is explained in mega ohms per centimeters.
2. Thermal property of the insulating materials
a) Specific heat thermal conductivity
In solid materials there is the wide variations of the properties though in the solids electrical and conductivity of thermal go together specially too close with the metals. When the liquids are utilized as a coolants in the transformers, these properties have little importance besides of the small range of the variations in all type insulating materials.
b) Thermal plasticity
Wound coils wires pressure is varies under the operating conditions cause of the contraction and expansion of parts and caused by the variations in the temperature. Practically pressure is generally accompanied through the slight vibratory consequent and motion abrasion, and this valuable to observe wire flow insulation at the high temperatures in the vibration absence.
c) Ignitability
Insulating materials are exposed to the arcing must be non-ignitable. When they are ignitable they must be self extinguishing, carbonization of materials.
d) Softening point
Solids softening points insulating materials must be above temperature occurred in practical.
e) Heat ageing
In fact ageing is wearing out of an insulating materials through removing its resistance to the mechanical injury. It increase quickly with the temperature, doubling for the every increase of the 10 degree C to 16 degree C, relying on the materials.
f) Thermal expansion
It is important because of mechanical effects reasoned by the thermal expansion besides temperature modifications. It should be small in insulating materials.
3. Chemical properties of the insulating materials
a) Resistance to the external chemical effects
Insulating materials must be oils resistant or gas fumes, alkalies, acids. Materials must not undergo oxidation and hydrolysis under adverse conditions.
b) Resistance to the chemicals I the soils
Cables are laid in soil can be deteriorate through the chemical action in the soils. Insulating materials suitability for this conditions can be decided through a long duration experience.
c) Water effect and tropical tests
Water is directly lowers to the electrical properties, like as dielectric strength and electrical resistance. Water may be transmitted by an outside coating and this is the cause of the inside damage. This may be absorbed through an insulating materials, this is the cause of the chemical modifications of the insulation itself or this may drastically lower with the surface resistance of an insulator.
4. Mechanical properties of insulating materials
a) Density
Electrical insulation is utilized on the basis of the volume and not weight, low density insulating is specially suitable for the small portable component and aircrafts component.
b) Viscosity
It is important in the liquid dielectric. Uniform viscosity gives uniform electrical and the thermal properties.
c) Moisture absorption
Water is lowers the dielectric strength and electrical resistance. With the absorption certain mechanical and chemical effects may get outcome. For example warping, swelling and corrosion.
d) Surface hardness
Surface hardness enables dielectric to the resist surface scratching and the abrasion when lower surface of the resistivity allows discontinue moisture films to the form and contribute to the corona and another surface deteriorating effects. Roughness of the surface is objectionable.
e) Surface tension
Low surface tension in liquid dielectrics is desirable because it causes greater electrical wetting components and so provides better cooling, greater voltage uniformity and impregnation. To enhance this property few wetting agent may be combined.
f) Uniformity
Dielectric must be uniform throughout as it contains electrical losses as the low as potential and electric-stresses uniform under the high voltage varies. When we study about the tensile strength, insulators, compressive strength, bending strength, shear strength and impact strength are important. Resistance and machinability to the splitting both are important.
Q2) What is static field ?
A2) Voltage is applied to the object like an electrical conductor, conductor goes to charged and forces to begin to act on the another charges in vicinity. There are two kinds of forces may be categorized are-
One is which arises from the stationary electric charges and know as the electrostatic force.
Another one which appears only when the charges are moving and known as magnetic force. An idea of the field has been developed to explain the existence and spatial distribution of the forces. Reference then made to the field of the force or easily electric and magnetic fields.
Q3) Define polar and non polar dielectric materials.
A3) Polar dielectric materials
These are made from the polar molecules. The mass center of the +ve charges does not coincide with the mass center of negative charges, in a molecule. When it is in a normal state, means no electric field applied every molecule has been some of the intrinsic permanent dipoles and contains an asymmetric form. The dielectric constant is considered as the permittivity ratio of the substance to free space permittivity. This is an expression of the extent to that a material concentration of the electric flux, is the electrical equivalent of the relative magnetic permeability.
Examples
Ammonia and HCI
Non-polar dielectric
These are made from the non-polar molecules. The center of the mass of +ve charges coincides with the mass center of negative charges in a molecule. When it is in normal state molecules contain zero dipoles and contains the asymmetric form.
Examples
Methane, benzene, etc.
Q4) What techniques used for the recycling of the insulating materials?
A4) For recycling of the recovering and using process for the old lagging materials old and waste materials are rock wool, aluminium silicate wool, ceramic fiber, glass wool, silicate cotton, and pearl stone. For recycling following steps are-
- Chemical dispersion- put into the solution which contain sulfonate surfactant or dispersion agent through waste and old explained fiber such as soak lagging materials which make it soften.
- Loosened by machinery- mixture which is got from step 1 brings out brute force through the mechanical tears and force, and further old and waste for explained fiber such as lagging material is loosened into the threadiness as explained step 1, step 2 bring out respectively and simultaneously.
- Mixture which is got by the step 2 is placed into the stirrer and by stirring method insulating becomes paste.
- Insulating pate which is got by the step 3 placed into the froth breaking pond froth breaking.
- After froth breaking the insulating paste is injected mould, put into the dry on the car together with the push and mould baking kiln dry. After having the packing, toasted and vanning are the composite microporous lagging materials finished products.
Q5) Define the reflection method of the measure the dielectric properties of materials?
A5) Transmission/reflection line method is a famous measurement method. In reflection method only basic waveguide mode (in coaxial line TEM mode and in waveguides TE mode) is assumed to the propagate. In this method transmission line concepts, in this a piece of the dielectric materials is put inside into the transmission line and electromagnetic wave is lead at sample. This technique provide disadvantages that frequencies above the 10 GHz are generally not measurable, besides of parasitic losses at the high frequencies. A measurement utilizing the reflection method includes placing a sample in the section of the waveguide or the coaxial line and also measure the two ports typical scattering parameters with the vector network analyzer. In this method includes measurements of reflected (S11 or S22) and transmission signal (S21 or S12). The related scattering parameters are related closely to typical permeability and permittivity of materials through equations.
Q6) Elobarate clausius mossotti equation?
A6) As discussed above the polarization can be considered as the to arise from the 3 major aspects like electronic, iconic and orientational polarization which is-
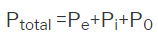
So, materials may be categorized into 3 types based on the dielectric behavior of the solids are concerned.
Elemental solid dielectric
These materials are consisting of single kinds of atoms like diamond and germanium. These all materials have neither ions nor permanent dipoles and so exhibit only electronic polarization.
Internal field Ei dielectric solids shows an electric dipole moment P
Which is 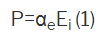
αe= electronic polarizability
n is consider the number of the molecules per unit dielectric volume, then polarization (P) is provided by
P=np
By substituting both equation
P=nαeEi (2)
Ei=E+P/3ε0 (3)
By the both equation put equation (3) in equation (2)
P=nαe[E+P/3 ε0] (4)
If £ is dielectric permittivity, D is displacement is given by
D= εE= ε0E+P
Or P={ ε- ε0)E
Or E=P/ε- ε0 (5)
By using equation (5) in equation (4), get
P=nαe[P/( ε- ε0)+ P/3 ε0]
= nαeP[2 ε0+ ε/3 ε0 (/ε- ε0)
Or 3 ε0/nαe= (ε+2 ε0)/( ε- ε0)
Or (ε- ε0)/(ε+2 ε0)=nαe/3 ε0 (6)
As ε0= εr ε0 (7)
By using equation (7) in (6), get
(εr ε0– ε0)/ (εr ε0+2 ε0)=nαe/3 ε0
Or ε0 (εr-1)/ ε0 (εr+2)= nαe/3 ε0
Or (εr-1)/ (εr+2) = nαe/3 ε0
This is the clausius Mossotti equation.
This equation displays the dielectric constants are determined by the n, αe, and γ.
Here γ=1/3.
Q7) Limitation of Clausius-Mossotti relation?
A7) Relation between microscopic property is called molecular polarizability and macroscopic property is named dielectric constant. This equation is valid for the non polar solid which have cubic crystal structure. There are some limitations of clausius mossotti equation are-
a) Polarization property is considered as the proportional to field.
b) Polar molecule is isotropic.
c) Short range interaction is absence.
Q8) Explain negative tan delta concept.
A8) Tan delta or dissipation factor is explained as reciprocal of the ratio between insulating materials capacitive reactance to the resistance at the specific frequency. The dissipation factor indicates insulation quality materials through the tangent ratio of resistive current IR to the capacitive current IC, negative tan delta factor would indirect a negative resistive current, that is physically impossible.
Q9) Explain parameters of the dielectric materials?
A9) Parameters of the dielectric materials are-
Dielectric constant
Dielectric constant also named relative permittivity specific inductive capacity, this is a property of the electrically insulating material and also equal of the capacitance of the capacitor filled with the given material of the capacitance of the identical vacuum capacitor without any dielectric material. Between the plates, the insertion of the dielectric, parallel plate capacitor always improves its capacitance, and ability to collect the opposite charges on every plate, also compared with this ability while the plates are separated from the vacuum.
If the capacitance value C filled with given dielectric and CO is the capacitance of the identical capacitor in the vacuum, this dielectric constant symbolized through the Greek letter Kappa, K, and expressed as K=C/CO.
Constant of the dielectric is a number without any dimensions.
In centimeter- gram- second system, the dielectric constant is identical to permittivity. It describes a large scale property of the dielectric without specifying electrical behavior on an atomic scale.
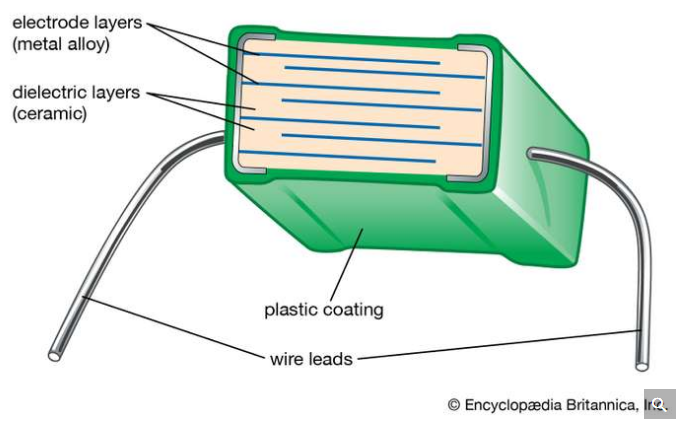
Dipole moment
When the charge is separate in any system the dipole moment arises. So, arise in the ionic bonds as well as in the covalent bonds. When the difference in the electronegativity between two chemical atoms is bonded, dipole moments occur.
The dipole moment is the measure of the chemical bond polarity between two atoms in a molecule. This is the concept of the electric dipole moment, this the measure of the separation of the positive and negative charges in the system.
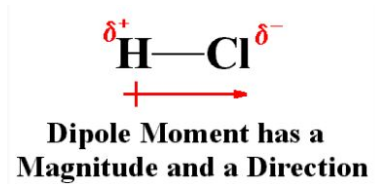
Dipole moment formula
Dipole Moment (µ) = Charge (Q) * distance of separation (r)
It is measured by the Debye unit which is denoted by the ‘D’, 1D=3.33564x10-30 C.m.
Where C is Coulomb and m is meter.
The bond dipole moment arises in the chemical bond between two atoms of the different type of electronegativities can be expressed as follows-
μ = 𝛿.d
Where μ is the bond dipole moment
𝛿 = magnitude of partial charges 𝛿+ and 𝛿–
And d = distance between 𝛿+ and 𝛿–
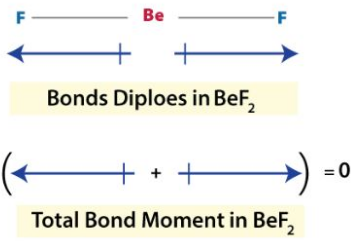
Example
The dipole moment of the Bef2
Polarization
When every unit of dielectric volume has been its electrical moment polarization occurs. There are two kinds of polarization are-
- When it occurs in the electric field.
- Spontaneously
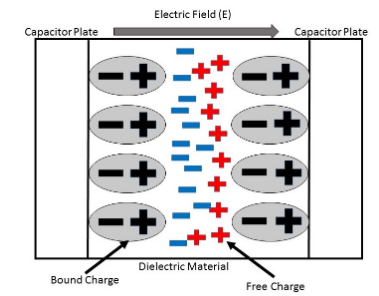
In some cases, mechanical stresses can also reason for the polarization. The dielectric constant is a parameter, qualities of the ability of some polarize materials. The term dielectric polarization explains the behavior of the material while an external electric field id applied to it.
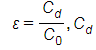
Cd= dielectric capacitance of medium,
C= subscript
O= vacuum dielectric capacitance
Dielectric polarization is followed by coherent charges on the surface of the dielectric. These coherent charges decrease the electric field inside the dielectric. Polarity is the quantitatively defined by the dielectric polarization.
Polarity is the vector quantity and measured by the
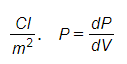
Polarizability
This is the measure of the ability of the material to become polarized in the presence of the applied electric field.
Polarization has occurred in polar or non-polar materials.
Q10) What is the complex dielectric constant of the materials?
A10) Complex dielectric constant is utilized to explain the dielectric constant when a periodic difference of the electric field, the field variations is explained through a sine-shaped waveform. And this is written in the form of ϵ=ϵ′+i⋅ϵ′
Where real part ϵ′, is permittivity element stored energy is quantified, part is directly proportional to field amplitude and imaginary part ϵ′′, is dielectric loss factor, that explains part of the electric energy which is lost by the movement of atoms as the outcome of regularly modifying field, contribution from this element is proportional to rate of electric field change, or first derivative of electrical field function. This element also provides phase rise difference between field function and polarization outcome. Without this element both would be the perfectly in the phase. Both elements relies on field frequency variation.