UNIT 3
Phase Diagram and Iron Carbon Diagram
Q1) Describe solid solutions and its types?
A1) Solid solutions:-
When homogeneous mixtures of two or more kinds of atoms (of metal) occur in the solid state, they are known as the solid solutions.
the more abundant atomic form is referred as solvent and less abundant atomic form is referred as solute.
For example:- In sterling silver (92.5% of silver of remaining is copper) is a solid solution of silver and copper where silver is solvent and copper is solute.
Types of solid solutions:-
Solid solutions are generally of two types
1) Substitutional solid solutions.
2) Interstitial solid solutions
Substitutional solid solution:- As suggested by the name substitutional means 'replacement’. here in this case when the atom of the parent metal or we can say atom of the solvent replaced by the atom of the solute metal then the solid solution is known as substitutional solid solution.
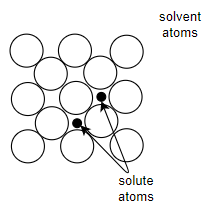
Interstitial solid solutions:-
In interstitial solid solutions the solute atom does not displace a solvent atom but rather it enters one of the holes or interstices between the solvent atoms.
1) For interstitial solid solutions solute and solvent should have similar electronegativity.
2) Intermetallic compounds are generally formed when one metal is stronger metallic and other is weakly metallic. Example:- 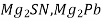
Q2) What is Hume Rothery rule?
A2) Hume Rothery rules defines us the various conditions under which an element court dissolve in a metal forming a solid solution.
These are as follows:
a) Crystal structure factor
b) Relative size factor
c) Chemical affinity sector
d) Relative valence factor
Crystal structure factor:- The crystal structure of solute and solvent must be similar. Example:- either crystal structure should be FCC, or BCC or HCP.
Relative size factor:- The atomic radius of the solute and solvent atoms must differ by not more than 15%. If it’s beyond 15% then it restrict solid solubility.
Chemical affinity factor:- When two metals have lesser chemical affinity then it forms solid solution. If there will be greater chemical affinity then it can cause compound formation. In others we can see that the solute and solvent should have similar electronegativity.
Relative valence factor:- The complete solubility occurs when solute and solvent must have same valency. A metal with lower valency is more likely to dissolve in metal of higher valency.
Q3) Explain nucleation and growth rate?
A3) Nucleation and crystal growth:-
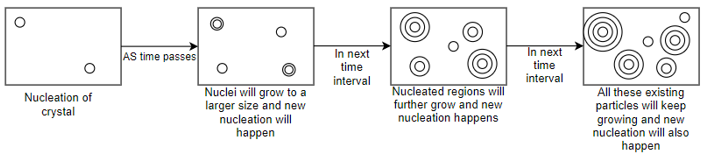
So, by above diagram we understand that gradually after increase of time process of nucleation and growth new tu nuclei are forming and already existing particles are also growing. So overall transformation rate will depend upon nucleation rate and growth rate.
Nucleation rate:- Number of Nutrition events per unit volume per second.
Denoted by 'I'
Unit – 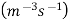
Growth rate:- Rate of increase of the size of the particle.
Denoted by 'G'.
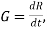 where R is particle size
where R is particle size
Unit – 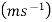
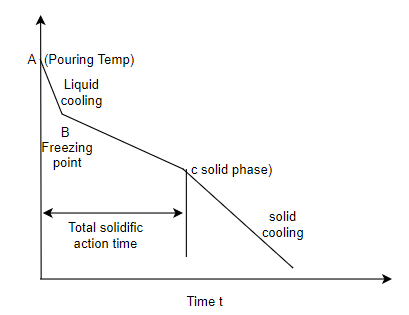
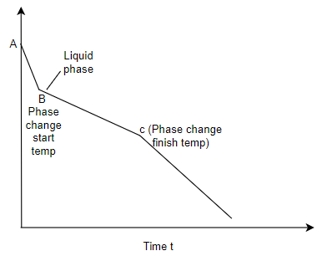
Q4) Differentiate in between solidification of metals and alloys with diagram?
A4) Solidification of metals and alloys:-
Total time taken from point A to point C is total solidification time.
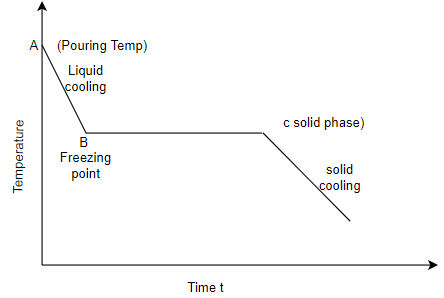
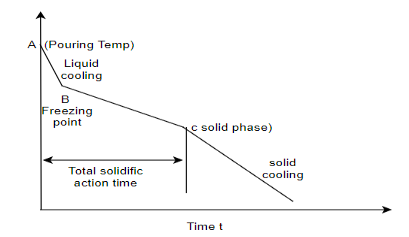
7. So the basic difference in between solidification of alloys and pure metals is the temperature difference as shown in above graphs, whereas pure metals solidify at constant temperature.
Q5) Explain phase diagram and its types?
A5) Phase diagram and types of phase diagram:-
Phase diagrams are used for quantitative description of the phase transformation and changes
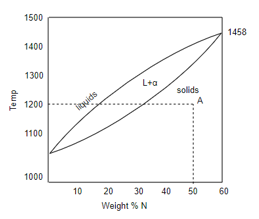
Here, the constitution point are Cu – Ni on the x axis which is expressed in terms of weight percentage of nickel whereas other thermodynamics variable is temperature on y axis.
As we have seen Cu-Ni phase diagram is very simple phase diagram. As you can see in the diagram there are two boundaries, the upper boundary is liquid boundary. At above the liquidus boundary there is liquid phase which is in equilibrium. And the lower boundary is solidus boundary and below this solid solution phase is stable, whitch is represented by 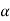 . And in between this there is
. And in between this there is 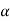 (two phase region).
(two phase region).
This phase diagram is in composition temperature space. So we mark any point in diagram, let us suppose 'A'. The X co-ordinate of this point is a composition. In this case if we want to find out the composition, so as shown in diagram it’s 50 weight percent of nickel is X co-ordinate and Y co-ordinate is 1200°C.
So this particular point represents an alloy A office Steve 8% of nickel held at 1200°C in equilibrium.
So such points are called constitution point. That means any point on the phase diagram can be constitution point.
Types of phase diagram
There are four main types of phase diagram
1) Isomorphous system
2) Eutectic system
3) Partial eutectic system
4) Layer system
A) Isomorphous system:-
Percentage amount of liquid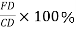
Percentage amount of solid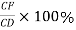
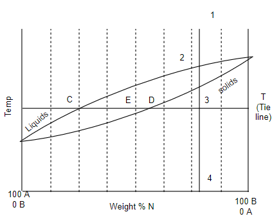
Cooling behavior of mixture
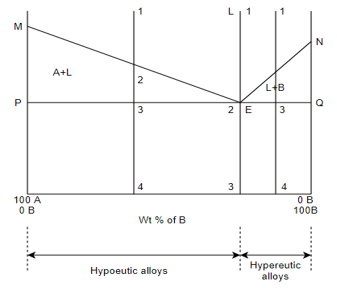
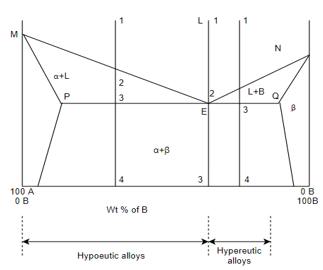
(B) Eutectic system:-
M - melting point of A
N - melting point of B
E - eutectic point
MEN – liquidus
MPEQN – solidus
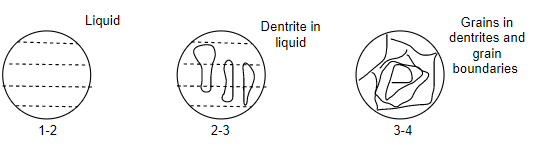
 Cooling behavior of eutectic alloys:-
Cooling behavior of eutectic alloys:-
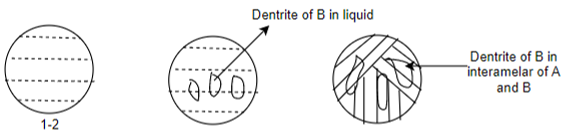
Cooling behavior of hypereutectic alloys:-

Cooling behavior of hypo eutectic alloys:-
(C) Partial eutectic system:
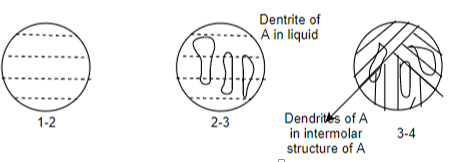
Two elements added will have the complete solubility in liquid and the partial solubility in solid state.
Example:- Ag-Cu, Pb-Sn, Sn-Bi, Pb-Sb
M - melting point of A, 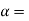 Solid solutes of B is A
Solid solutes of B is A
N- melting point of B, 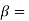 Solid solutes of A is B
Solid solutes of A is B
MNEQN – solidus
MEN – liquidus
(D) Layer system:-
Two elements added are completely in soluble in liquid as well as in solid state.
Example:- Ag-Fe, Cu-Tu, Cu-Me
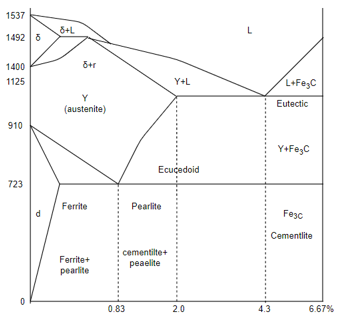
Q6) Explain Fe-C equlibrium phase diagram?
A6) Fe-C Equilibrium phase diagram
Eutectic point 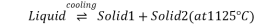
Eutectoid point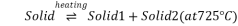
Peritectic point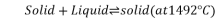
Peritectoid point 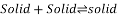
Q7) Define Gibb’s phase rule?
A7) Gibb’s phase rule:-
P+F =C+2
Where, P is number of phases
F is degree of freedom and
C is number of components
Degree of freedom:- It is the total number of independent or intrinsic or extrinsic variables are required to locate or fix the state of any system is known as degree of freedom.
Example:- suppose we have a system maybe water.
Now we have to find out degree of freedom ?
Ans. Case 1:- 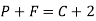
Here, P = 1 (only 1 phase that is liquid)
F = ?
C = 1 (only water)
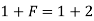
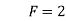
Case 2:- If there will be ice and water, then
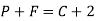
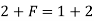
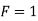
Case 3:- When P = 3 (solid liquid gas)
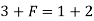
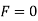
Q8) Define terms degree of freedom and triple point?
A8) Degree of freedom:- It is the total number of independent or intrinsic or extrinsic variables are required to locate or fix the state of any system is known as degree of freedom.
Triple point:- The temperature and pressure at which substance can exist in equilibrium in the liquid, solid and gaseous state.
Q9) Describe cooling curves for alloys and pure metals?
A9) Cooling curve for pure metals:-
Under equilibrium conditions, all metals exhibit a definite melting or freezing point as shown in above diagram.
Cooling curve of alloys:- In this method alloys with different compositions are melted and then the temperature of the mixture is measured at certain time intervals while cooling back to room temperature.
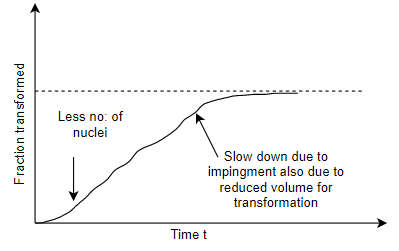
Q10) Describe nucleation graphically with respect to time?
A10) As you can see the friction transforms can take a maximum value of 1, which means 100% transformation has happened and the minimum value will be zero at beginning.
So, initially since nucleation is about to happen and there is no nucleus so overall transformation rate would be 0.
Then as nucleation started there will be few nuclei as shown in figure then after the rate will pick up. but when it reaches at the end of the transformation it will again slowed down as shown in graph. As when it reaches to 1 the overall transformation rate slowdown.
If we will allow for the growth the particle will start impinge among each other.