UNIT 5
Ferrous Material
Q1) How are you Engineering materials classified?
A1) The engineering materials can broadly be classified as:
a) Ferrous Metals
b) Non-ferrous Metals (aluminum, magnesium, copper, nickel, titanium)
c) Plastics (thermoplastics, thermosets)
d) Ceramics and Diamond
e) Composite Materials &
f) Nano-materials.
The engineering materials are often primarily selected based on their mechanical, physical, chemical and manufacturing properties.
Q2) How ferrous metal differ from non ferrous metal?
A2) In case of ferrous (ferrum=iron) metals, the base metal is iron. They compose a large part of the overall metals in use today. This is made possible by their properties that suit many different industries .
Non-ferrous metals, on the other hand, do not include iron. This distinction is made because it brings along a certain characteristic change that non-ferrous metals do not provide. Non ferrous metals are those which have a metal other than iron as there main constituent such as copper, aluminium, brass, bronze, silver etc.
Q3) How cast iron differ from Steel?
A3) The primary difference between cast iron and steel is the carbon content. They are both types of iron alloys. Cast iron has a carbon content great than 2%; it is a combination of iron-carbon alloys with carbon content. Steel contains less than 2% of carbon content with many steel alloys having less than 1%.
Cast iron is harder and stronger but it’s not as tough as steel. Steel is tougher but it’s not as hard and strong as cast iron.
Steel has ductile and malleable properties and its melting point is higher than cast iron. Cast iron is characterized by: higher fragility; lower toughness; higher cast ability, higher stability at higher temperatures; lower cost; lower machinability; lower electric conductivity; lower plasticity; lower co-efficient of friction, lower density, and lower thermal conductivity.
Cast iron (usually, but not always) compared to steel has:
An iron alloy with an amount of carbon superior to 2,11% is considered cast iron (whereas steels have an amount of carbon inferior to 2.11%). The most notable difference in the microstructure is that cast iron has graphite inclusions.
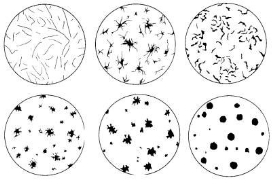
The image shows different types of graphite inclusions in cast steel, from acicular (top left) to nodular (bottom right). The shape is determined by the amount and type of alloying elements and the heat treatment. The presence of graphite determines the differences in properties with steel.
Q4) Write short note on:
a) Grey cast iron
b) White cast iron
c) Ductile cast iron
d) Malleable cast iron
A4) A) Grey cast iron:- Grey cast iron is grey in color which is due to the carbon being principally in the form of graphite. It contains
C = 2.5 to 3.6%
Si = 1.1 to 2.8%
Mn = 0.4 to 1.0%
P = less than 0.15%
S = less than 0.1 %
Fe = remaining
B) White cast iron:- The white colour is due to the fact that the carbon is thin iron in in combined form as iron carbide which is commonly specified as cementite. It is the hardest constituent of iron
C = 3.2 - 3.6%
Si = 0.4 – 1.1%
Mg = 0.1 – 0.4%
P = less than 0.3 %
S = less than 0.2 %
Fe = remaining
C) Ductile cast iron:
When small quantities of magnesium or cerium is added to cast iron then graphite content is converted into nodular or idle form and it is well dispersed throughout the material.
Carbon = 3.2 % 4.2 %
Silicon = 1.0 – 4.0 %
Magnesium= 0.1 – 0.8%
Nickel = 0.0 – 3.5%
Manganese = 0.5 – 0.1%
Iron = remaining
D) Malleable cast iron :- malleable cast iron that easily workable. It typically created using heat treatment processes on white cast iron. the white cast iron is heated for 1 to 2 days after which it is cooled, when finished. Malleable cast iron can be bent and manipulated to achieve unique shape and sizes.
C = 2.5%
Si = 1.0 %
Mn = 0.55%
Iron = remaining
Q5) What are alloy steels?
A5) Alloy Steel that is alloyed with a variety of elements in total amount between 1.0 % and 50% by weight to improve its Mechanical properties. Alloy steel characterized in in two parts as per alloy percentage.
1) High alloy Steel
2) Low alloy Steel
High alloy Steel: High alloy Steel are defined by a high percentage of alloying elements. Its most common high alloy steel is stainless steel which contains at least 12% chromium. Stainless steel is generally split into three basic types which are martensite, territic and austenitic.
Low alloy Steel:- Low alloy steels have a much lower percentage of alloying elements usually 1to 5%, these steels have very different strains and uses.
Q6) What are plain carbon steels? discuss in brief the classification of plain carbon steels and also state few application of different plain carbon Steel?
A6) Carbon steel is a steel with carbon content from about 0.05to 2.1 % by weight.
Classification of carbon Steel
Typically classification of carbon Steel in 4 part according to carbon content:
Low carbon Steel-
Typically contains 0.05% to 0.30 % carbon content. Sometimes is known as mild steel also which has tensile strength of 555 N/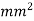 and hardness of 140 BHN.
and hardness of 140 BHN.
Medium carbon Steel:-
Typically has a carbon range of 0.30 % to 0.60 % and a manganese content ranging from 0.06% to 1.65 %. This product is stronger than low carbon Steel.
High carbon Steel:-
Commonly known as carbon tool Steel. it typically has a carbon range of between 0.60 % and 1.50% very strong used for springs tools and high strength wires.
Ultra high carbon Steel:-
Approximately 1.50 – 2.0% carbon content Steel that can be tempered to great hardness. Used for special purposes like (non-industrial purpose) knife, axles or punches.
Industrial application:- The industrial application of carbon steel:
Q7) Explain the effect of alloying element?
A7) Steel is a combination of iron and carbon. Steel is alloyed with various element to improve physical properties.
1) Carbon:- The most common important constituent of Steel is it raises tensile strength, hardness and resistance to wear and abrasion. It lowers ductility, toughness and machinability.
2) Chromium (Cr):-; Increase tensile strength, hardness, toughness resistance to wear and abrasion, resistance to corrosion and scaling at elevated temperature.
3) Columbium (CB):- Used as stabilizing element in stainless steel each has a high affinity for carbon and forms carbides which are uniformly dispersed throughout the Steel.
4) Copper (Cu):- It significant amount is detrimental to hot working Steel. Copper negatively affects forge welding. Copper can be detrimental to surface quality.
5) Manganese (MN):- A deoxidizer and degasifier and reacts with Sulphur to improve forge ability. It increase tensile strength, hardness, hardenability and resistance to wear.
6) Molybdenum (MO):-; increases strength, hardness, hardenability and toughness as well as creep resistance and strength at elevated temperature.
7) Nickel (Ni):- Increases strength hardness without ductility and toughness. It also increase resistance to corrosion.
8) Phosphorus (P):- increase strength and hardness and improve machinability however it adds mark brittleness or cold shortness to Steel.
9) Silicon (Si):- deoxidizer and degasifier increases tensile and yield strength, hardness, forge ability E and magnetic permeability.
10) Sulphur (S):- improve machinability in free cutting steels but without sufficient manganese. It produce brittleness at red heat.
11) Titanium (Ti):- improve strength and corrosion resistance limits austenite grain size.
12) Tungsten (W): increase hardness. Particularly at elevated temperature due to stable carbides, refines grain size.
13) Vanadium (V):- increase strength, hardness, creep resistance, and impact resistance due to formation of heart vanadium carbides.
Q8) What did you understand about designation of Steel?
A8) Carbon steel and alloy Steel are designed a four digit number, where by the first digit indicates the main alloying element, the second digit indicates top grid element and the last two digit indicate the amount of carbon, in hundreds of percent by weight. For example a 1060 steel is plain carbon steel containing 0.60 weight percent C.
The 'H' suffix can be added to any designation to denote hardenability.
Major classification of Steel
SAE designation | Type |
1 xxx | Carbon Steel |
2xxx | Nickel Steel |
3xxx | Nickel chromium Steel |
4xxx | Molybdenum Steel |
5xxx | Chromium Steel |
6xxx | Chromium vanadium Steel |
7xxx | Tungsten Steel |
8xxx | Nickel chromium molybdenum Steel |
9xxx | Silicon manganese Steel |
Carbon and alloy Steel grades
10xx | Plain carbon (Mn 1.00% ) |
11xx | Resulphurized |
12xx | Resulphurized and rephosphorized |
15xx | Plain carbon (Mn 1.50-1.65%) |
Q9) Discuss in brief the effect of impurities in cast iron?
A9) Alloy cast iron is obtained by adding the different constituents to gain different Mechanical properties like strength, Wear resistance, corrosion resistance, Heat resistance. Those are like nickel, chromium, molybdenum and copper. The following are the different impurities which will affect the mechanical characteristics of the Cast Iron.
These are the common impurities which will affect the properties of Cast Iron. Let’s discuss them in brief one by one.
Silicon
For the purpose of soft and easy machinability, we should maintain the proper amount of silicon in cast iron. In Cast Iron, it is already present up 4% as one of the constituents. We can add more silicon further to Cast Iron to increase the desired properties. The main effect of the further adding silicon to the cast iron is Increases fluidity for casting, Influences the solidification of liquid alloys.
Sulphur
The brittleness in the Cast Iron can be increased by adding the Sulphur. But sometimes it should maintain a bit low due to too much sulphur gives unsound casting. For the foundry purposes, it should keep under 0.1%.
Manganese
Manganese makes the Cast Iron looks white and increases the hardenability and tensile strength. The amount of manganese in cast iron is up to 0.75%. Manganese used to counters the brittleness from sulphur in the Cast Iron.
Phosphorus
Phosphorus may occupy up to 1% in cast iron Composition. Due to phosphorus present in cast iron, it will increase the fluidity of cast iron which helps for Intricated (Complex and detailed) casting designs.
Q10) Write short note on
A10) Stainless steel:- Stainless Steel contains chromium together with nickel as alloy and rest is iron. It has been defined as that Steel which when correctly he treated and finished. Resist oxidation and corrosive attack from most corrosive media. Stainless steel surface is responsible for corrosion resistance. Minimum chromium content of 12 percentage required for the films formation. 12 percentage sufficient to resist the most severe atmospheric corrosive conditions. Additional of nickel improves ductility and imparts strength. Addition of molybdenum improves its resistance to sulfuric, sulfuric acid and organic acids. Addition of manganese increase hot workability of the Steel.
High speed Steel: High-speed steel (HSS or HS) is a subset of tool steels, commonly used as cutting tool material. Tool steel is a variety of carbon steel and alloy Steel that are particularly well suitable to be made into tools. Their sustainability comes from their distinctive hardness, resistance to abrasion and D formation and their ability to hold a cutting edge at elevated temperature. Tool steels are suited for use in the shipping of other materials with a carbon content between 0.5% – 1.5%, tool Steel are manufactured under carefully controlled condition to produce the required quantity.
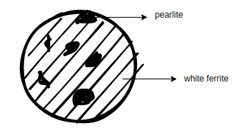
Microstructure of Steel: Here we have specified some material (microstructure and properties)
Mild steel
Physical properties: - Some mild steel's physical properties are as follows
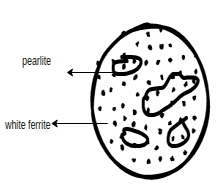
Physical properties:- Some low carbon steel properties are as follows
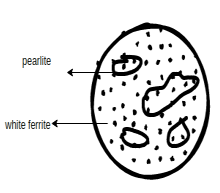
2.High carbon Steel
Physical properties:- High carbon steel physical properties are as follows