UNIT 1
Fundamentals of Thermodynamics
Q1. Explain the term System and different types of systems.
A1) System
Thermodynamic system is the region which is under observation for thermodynamic changes.
There are 3 types of systems thermodynamics
- Isolated systems,
- Closed system, and
- Open system
An isolated system cannot exchange either mass or energy with the surroundings.
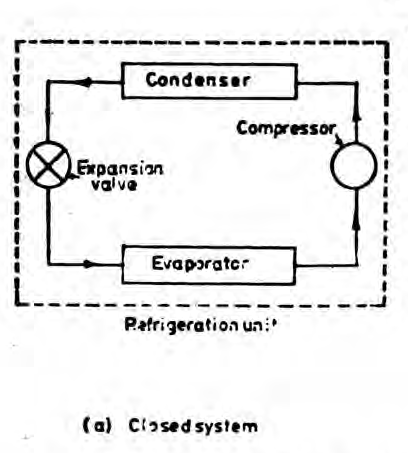
A closed system exchanges energy with the surrounding but mass transfer is not possible.
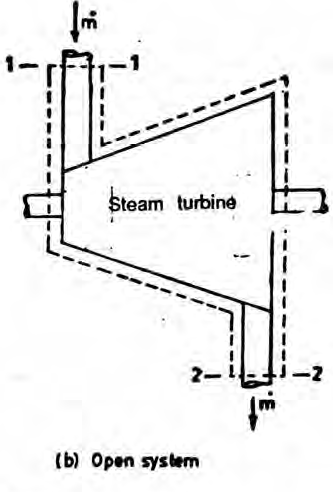
An open system can exchange both mass and energy with the surroundings.
Q2) Explain the Zeroth law of thermodynamics.
A2) Zeroth Law of Thermodynamics:
If Body 1 is in thermal equilibrium with Body 3 and Body 2 is in thermal equilibrium with Body 3, it implies that Body 1 is also in thermal equilibrium with Body 2.
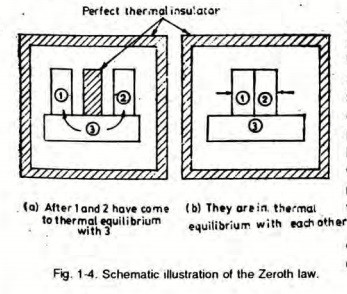
Thermal equilibrium is a state where no heat is transferred between two bodies in contact or between system and surrounding.
Q3) Explain the First law of thermodynamics.
A3) First Law of Thermodynamics:
This law is the same as the law of the conservation of energy, which states that energy can neither be created nor destroyed if mass is conserved. The sum total of the energy in the universe is constant. Energy, however, can be converted from one form into another form. This is the thermodynamic aspect of first law. A machine cannot create work from nothing nor it can deliver more work than it receives. In a steam generating plant, the chemical energy of the fuel is converted into heat energy in the boiler, which in turn is converted into mechanical work in the steam engine or steam turbine. If the turbine is coupled to an electric dynamo, the mechanical energy is converted into electrical energy. If the dynamo is supplying the electrical energy produced by it to drive an electric motor, the electrical energy is again converted into mechanical energy. It was established by Joule that heat and mechanical energies are mutually convertible. Heat requires for its production; a definite number of units work for each unit of heat produced. Similarly, heat produces by its disappearance, a definite number of units of work for each unit of heat converted. This is known as the first law of thermodynamics. Joules experiments showed that for a closed system during a cyclic process, the sum of the work transferred is equal to the sum of the heat transferred. Mathematically, it is written as
∮dW= ∮dQ
The circle on integral sign represents a cyclic process. This means during any cycle, a closed system executes, the cyclic integral of work is equal to cyclic integral of heat. Work and heat both being measured in joules (J) or kilojoules (kJ).
For a non-cyclic process, a closed system (In absence of KE and PE) executes, the work transferred and heat transferred may not be equal, and the difference between the two is accounted for by a change in internal energy, u of the system. This can be stated mathematically as
Q - W = Δu
Q = Δu + W
Q4) Explain the Joule’s experiment.
A4) The main aim of Joules Experiment was to determine the relationship between the work done and the quantity of heat produced in mechanical system.
Construction and Working:
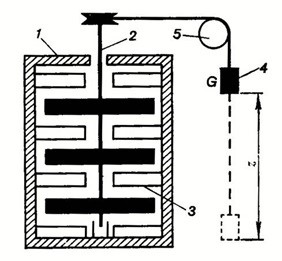
Importance of Joule’s Experiments:
Q5) Explain the PMM1.
A5) Perpetual motion machine of first kind (PMM1)
It is an imaginary concept. This would violate the first law of thermodynamics. It produces energy without any input and as we know that according to the law of energy conservation, energy cannot be created or destroyed but can only be converted from one form of energy to other form of energy.
Therefore, a machine that does not follow the first law of thermodynamics or law of conservation of energy is termed as perpetual motion machine of first kind i.e. PMM1
Q6) Apply SFEE to boiler and derive the expression.
A6) A boiler is a device used for steam generation at constant pressure.
Heat is supplied to boiler drum externally by combustion of fuel in presence of air. Products of combustion are called Flue gases.
For a boiler - W =0, ΔK.E.=0 and Δ P.E.=0
On using these conditions S.F.E.Equation,
Q = m (h2 - h1)
Q7. Apply SFEE to nozzle and derive the expression.
A7) In case of a nozzle as the enthalpy of the fluid decreases and pressure drops simultaneously the flow of fluid is accelerated. This is generally used to convert the part of the energy of steam into kinetic energy of steam supplied to the turbine.
For this system, ∆PE=0,W=0,Q=0
Applying the energy equation to the system,
h1+(C12/2)=h2+(C22/2)h1+(C12/2)=h2+(C22/2)
Q8) Air enters a compressor at 105 Pa and 25°C having volume of 1.8 m3/kg and is compressed to 5 × 105 Pa isothermally.
Determine:
(i) Work done;
(ii) Change in internal energy; and
(iii) Heat transferred.
A8) For isothermal process :
(i) W1-2= 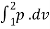 = p1v1
= p1v1 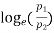
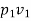 =
= 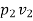 for isothermal process
for isothermal process
W1-2 = 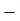 105
105 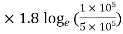
= 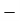 2.897
2.897 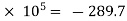 kJ/kg.
kJ/kg.
( - ve sign indicates that the work is supplied to the air)
 Work done on the air = 289.7 kJ/kg.
Work done on the air = 289.7 kJ/kg.
(ii) Since temperature is constant,
 u2 – u1 = 0
u2 – u1 = 0
(iii) Again, Q1 – 2 = ( u2 – u1 ) + W
= 0 + ( - 289.7) = - 289.7 kJ
( - ve sign indicates that heat is lost from the system to the surroundings)
 Heat rejected = 289.7 kJ/kg.
Heat rejected = 289.7 kJ/kg.
Q9) 1 kg of gaseous CO2 contained in a closed system undergoes a reversible process at constant pressure. During this process 42 kJ of internal energy is decreased. Determine the work done during the process. Take Cp = 840 J/kg°C and Cv = 600 J/kg°C.
A9) Mass CO2, m = 1 kg
Decrease in internal energy, 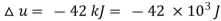
Specific heat at constant pressure, 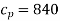 J/k
J/k
Specific heat at constant volume, 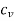 = 600 J/k
= 600 J/k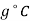
Let, initial temperature of CO2 = T1
Final temperature of CO2 = T2
Now change in internal energy,
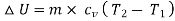
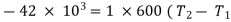 )
)
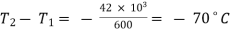
The heat supplied or rejected,
Q = mcp ( T2 – T1)
= 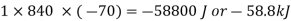
Applying first law to the process,
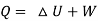
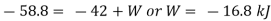
 Work done during the process = - 16.8 kJ.
Work done during the process = - 16.8 kJ.
Q10) 0.2 m3 of air at 4 bar and 130°C is contained in a system. A reversible adiabatic expansion takes place till the pressure falls to 1.02 bar. The gas is then heated at constant pressure till enthalpy increases by 72.5 kJ.
Calculate:
(i) The work done;
(ii) The index of expansion, if the above processes are replaced by a single reversible polytropic process giving the same work between the same initial and final states.
Take Cp = 1 kJ/kg K, Cv = 0.714 kJ/kg K.
A10)
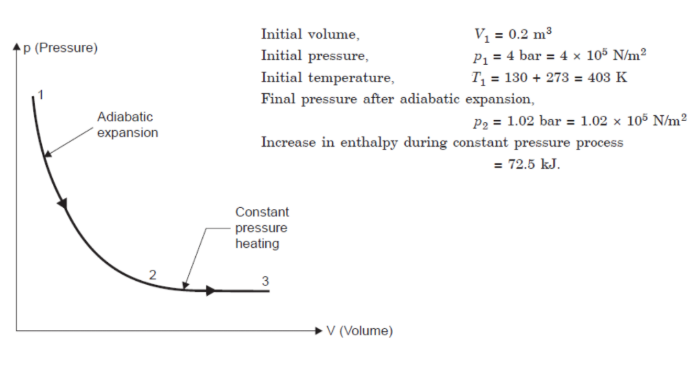
(i) Work done :
Process 1-2: Reversible adiabatic process :
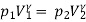
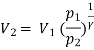
Also 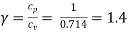

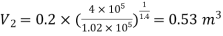
Also, 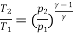
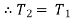 (
(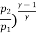
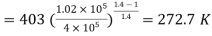
Mass of the gas,
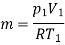
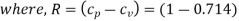 kJ/kg K
kJ/kg K
= 0.286 kJ/kg K = 286 J/kg K or 286 Nm/kg K
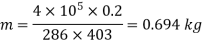
Process 2-3. Constant pressure :
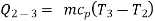
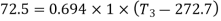
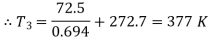
Also, 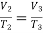
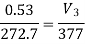
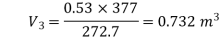
Work done by the path 1-2-3 is given by
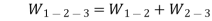
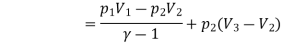
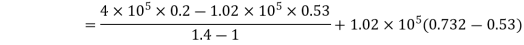
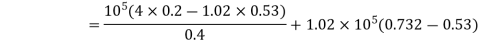
= 64850 +20604 = 85454 Nm or J
Hence, total work done = 85454 Nm or J.
(ii) Index of expansion, n :
If the work done by the polytropic process is the same,
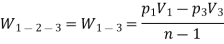
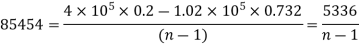
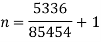
n= 1.062
Hence, value of index = 1.062