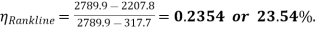UNIT 4
Properties of Pure substances & Thermodynamics of Vapour
Q1) Explain the various steps and heats involved in formation of steam.
A1) Formation of steam
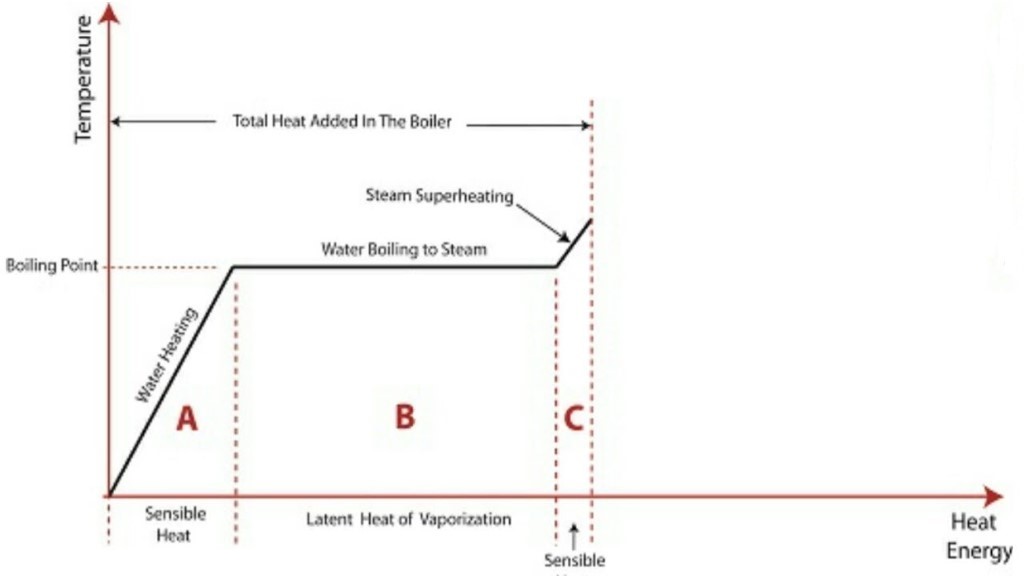
The above diagram shows the phase change occurring when water from and at 0°C is heated till is converted to steam and further to superheat it.
Temperature is plotted as ordinate and Enthalpy as abscissa.
Phase Change
hsup= hg + Cp (tsup - tsat)
tsup = temperature of superheated steam
tsat = temperature of saturation of steam
Cp = Specific heat of water at constant pressure.
Q2) Explain the critical point of water with the help of T-v diagram.
A2) 1. T-v diagram for pure substance:
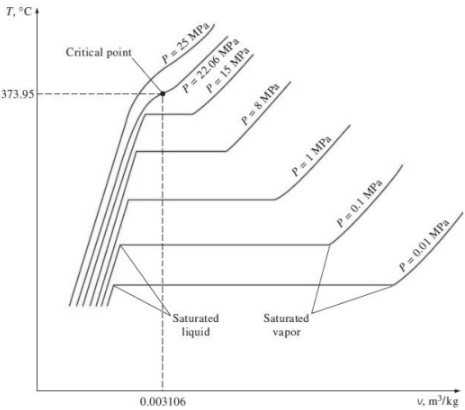
Critical Point
Q3) Explain triple point of water.
A3) Triple point
Q4) Define dryness fraction.
A4) Dryness fraction is defined ratio of mass of dry steam to total mass of mixture of wet steam.
It is denoted by ‘x’.
It is very important property of steam and needs to be determined before the steam is fed to the boiler.
Q5) Explain the Simple Rankine Cycle.
A5) Simple Rankine Cycle:
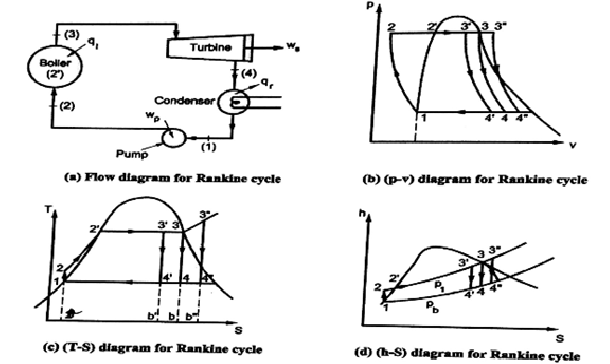
The above diagrams illustrate the various processes of a simple Rankine Cycle applied to a simple steam powered power plant.
Let us the various processes.
Analysis of Rankine Cycle
Assume 1 kg of steam in the cycle and applying SFEE to various processes, we get,
for Process 1-2
Pump work = h2 - h1 = ʃ-V dp
for Process 2-3
Heat supplied in boiler (qi) = h3 - h2
For Process 3-4
Work done in turbine = WT= h3 - h4
For Process 4-1
Heat rejected in condenser (qr) = h4 – h1
Efficiency of Rankine Cycle (η)
Efficiency of Rankine Cycle η = (Shaft work) / (Heat supplied)
= WT – WP / qi
= {(h3 - h4) - (h2 - h1)} / h3 - h2
Q6) Explain the modified Rankine cycle.
A6) 1. Modified Rankine Cycle – Rankine Cycle with Reheat
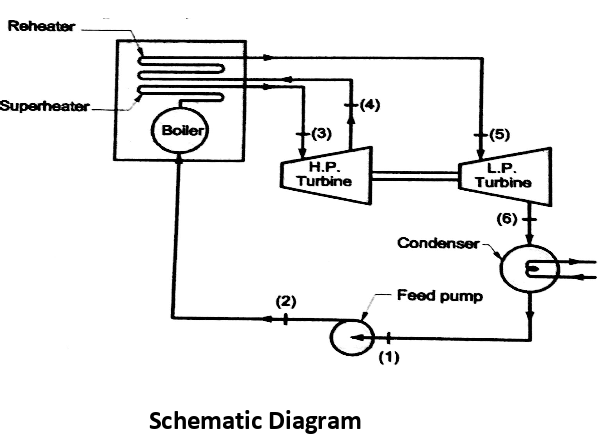
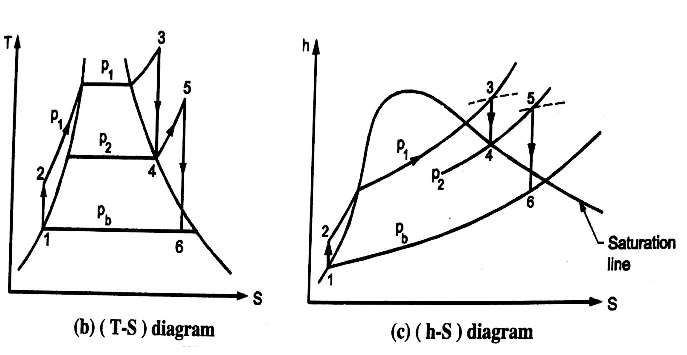
Analysis of Rankine cycle with reheat
Efficiency of reheat cycle for two stage turbines with one reheater in between the stage can be computed as
WT = WT(HP) + WT(LP)
= (h3 – h4) + (h5 – h6)
Wp= (h2 - h1)
Heat Supplied = Heat supplied in boiler and reheater = (h3 - h2) + (h5 - h4)
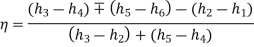
Advantages
Disadvantages
Q7) Describe the working of throttling calorimeter.
A7) 1. Throttling Calorimeter
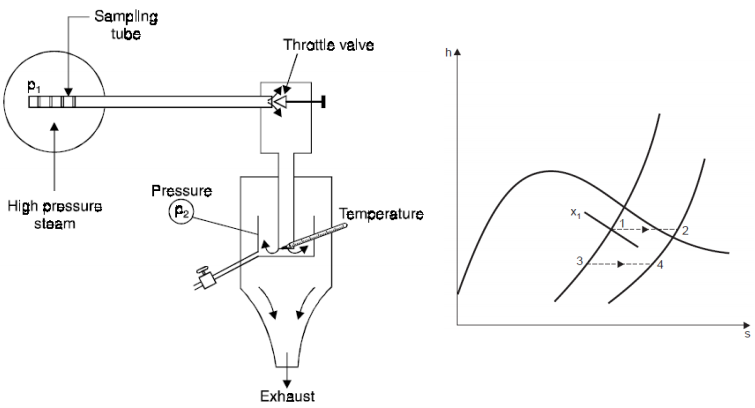
h2 = h1 = hf1 + x1hfg1
x1 = (h2 - hf1) / hfg1
Q8) A throttling calorimeter is used to measure the dryness fraction of the steam in the steam main which has steam flowing at a pressure of 8 bar. The steam after passing through the calorimeter is at 1 bar pressure and 115°C. Calculate the dryness fraction of the steam in the main.
Take Cps = 2.1 kJ/kg K.
A8) Condition of steam before throttling :
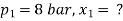
Condition of steam after throttling :
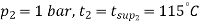
As throttling is a constant enthalpy process
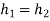
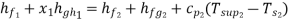
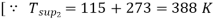
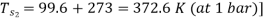
720.9 + x1 2046.5 = 417.5 + 2257.9 + 2.1(388 – 372.6)
720.9 + 2046.5 x1 = 2707.7
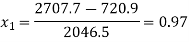
Q9) Calculate the internal energy per kg of superheated steam at a pressure of 10 bar and a temperature of 300°C. Also find the change of internal energy if this steam is expanded to 1.4 bar and dryness fraction 0.8.
Q9) At 10 bar, 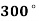 C. From steam tables for superheated steam.
C. From steam tables for superheated steam.
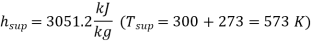
And corresponding to 10 bar (from tables of dry saturated steam)
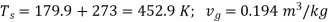
To find 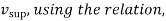
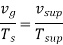
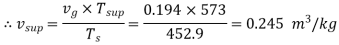
Internal energy of superheated steam at 10 bar,
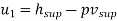
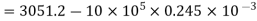
= 2806.2 kJ/kg. (Ans.)
At 1.4 bar. From steam tables :
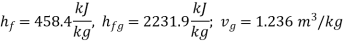
Enthalpy of wet steam (after expansion)
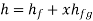
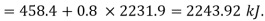
Internal energy of this steam,
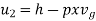
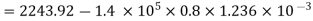
= 2105.49 kJ
Hence change of internal energy per kg
u2 - u1 = 2105.49 – 2806.2
= - 700.7 kJ. (Ans.)
Q10) In a steam power cycle, the steam supply is at 15 bar and dry and saturated. The condenser pressure is 0.4 bar. Calculate the Carnot and Rankine efficiencies of the cycle. Neglect pump work.
A10) Steam supply pressure, p1= 15 bar, x1= 1
Condenser pressure, p2 = 0.4 bar
Carnot and Rankine efficiencies :
At 15 bar : 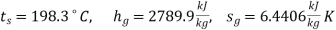
At 0.4 bar : 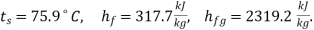
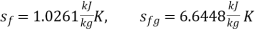
T1 = 198. 3 + 273 = 471.3 K
T2 = 75.9 + 273 = 348.9 K
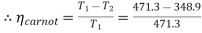
= 0.259 or 25.9%. (Ans.)

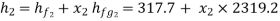
Value of x2 :
As the steam expands isentropically,

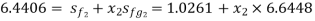
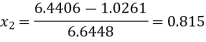
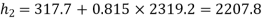 kJ/kg
kJ/kg
Hence,