EC
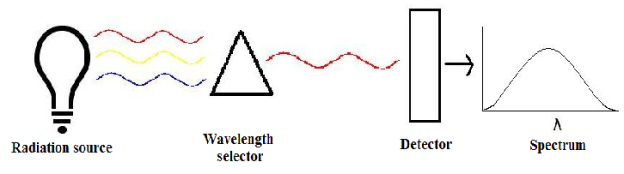
UNIT – II INSTRUMENTAL METHODS OF CHEMICAL ANALYSIS Q1) What is the advantage of instrumental method?The minute sample quantity is required. The instrumental method determination is considerably fast. Complex mixture can be analysed either with or without their separation. Sufficient reliability and accuracy of results are obtained by instrumental method. When non-instrumental method is not possible, instrumental method is the only answer to the problem. Q2) What is the disadvantage of instrumental method?A2)Instrumental methods are costly because of cost, maintenance and trained personnel required for their handling. The used instrument defines sensitivity and accuracy. Specialized training for handling instrument is required. There is frequent need of checking results with other methods. In some cases, instrumental method may not be specific. Q3) What is spectrometry?A3) A spectrometer is used to measure wavelengths of electromagnetic radiation that has interacted with a sample. Incident light can be reflected off, absorbed by, or transmitted through a sample. The way the incident light changes during the interaction with the sample is characteristic of the sample. A spectrometer measures this change over a range of incident wavelengths.
There are three main components in all spectrometers; these components can vary widely between instruments for specific applications and levels of resolution. Generally, these components produce the electromagnetic radiation, somehow narrows the electromagnetic radiation to a specified range, and then detect the resulting electromagnetic radiation after is has interacted with the sample. Q4) What is electromagnetic radiation?A4) Electromagnetic radiations are a form of energy that is transmitted through space with high velocity. Electromagnetic radiations consist of discrete packets of energy which are called photons. A photon consists of an oscillating electric field (E) and an oscillating magnetic field (M). Q5) Explain Lambert’s law.A5) The rate of decrease in intensity of radiation is directly proportional to path length of solution when the monochromatic light passes through a solution of constant concentration.Mathematically the law can be written as,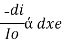 Therefore,
Therefore, intensity (radiant power) of the incident radiation.
intensity (radiant power) of the incident radiation.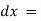 small thickness of solution or path length.
small thickness of solution or path length.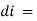 small decrease in intensity of light
small decrease in intensity of light 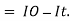 Intensity or radiant power is the number of photons per unit area per second
Intensity or radiant power is the number of photons per unit area per second 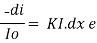 Integrating within limits
Integrating within limits 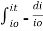 =
= 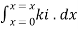 Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be
Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be 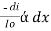 -- --------------------- (lamberts law for constant concentration)
-- --------------------- (lamberts law for constant concentration)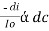 ----------------------- - - (beers law for constant thickness of solution)Therefore,
----------------------- - - (beers law for constant thickness of solution)Therefore,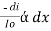 or
or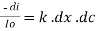 Integrating within limits Ln
Integrating within limits Ln Or
Or
Log 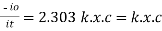 Therefore, K is absorptivity constantThe term log
Therefore, K is absorptivity constantThe term log 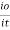 is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution (
is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution ( ) and unit length of path (
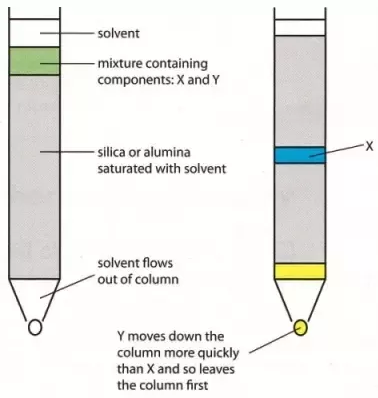
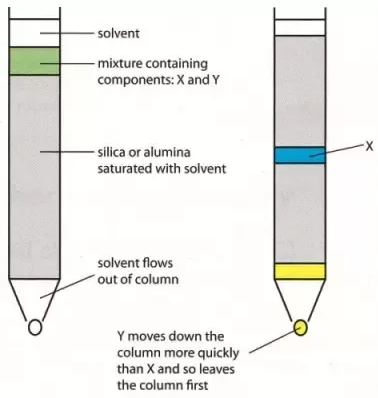
) and unit length of path (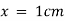 ) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
Q8) What is Adsorption chromatography?A8) This chromatography is one of the oldest type of chromatography. It utilizes a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibrium between the mobile and stationary phase accounts for the separation of different solutes.
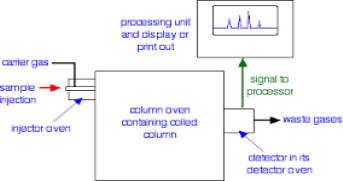
Q9) Explain gas liquid chromatograph.A9) This is the one of the fastest separation method, which allows for the quantitative determination of each compound of the mixture. In the Gas-Liquid chromatography the process begins by the bringing of sample by the syringe to the heated injection chamber. The sample is passing through the nitrogen gas in the column. The same column is has the thin film of liquid. After the separation of the carried gas the separated mixture flows in detector which activates a recorder. Traces a series of peaks on chromatogram.
Q10) What are the applications of chromatography?A10)1) The chromatographic technique is used for the separation of amino acids, proteins and carbohydrates.2) It is helpful for the qualitative and quantitative analysis of complex mixtures.3) It is also used for the analysis of drugs, hormones, vitamins and brain amines.4) This technique is also helpful for the determination of molecular weight of proteins.5) It is helpful in detection of phenolic compounds in drinking water.6) It is helpful in detection of endogenous Neuro peptides in extracellular fluid of brain etc.
A1) Advantages of instrumental methods:-
|
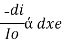 Therefore,
Therefore, intensity (radiant power) of the incident radiation.
intensity (radiant power) of the incident radiation.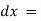 small thickness of solution or path length.
small thickness of solution or path length.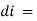 small decrease in intensity of light
small decrease in intensity of light 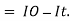 Intensity or radiant power is the number of photons per unit area per second
Intensity or radiant power is the number of photons per unit area per second 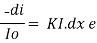 Integrating within limits
Integrating within limits 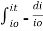 =
= 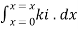 Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be
Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be 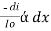 -- --------------------- (lamberts law for constant concentration)
-- --------------------- (lamberts law for constant concentration)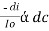 ----------------------- - - (beers law for constant thickness of solution)Therefore,
----------------------- - - (beers law for constant thickness of solution)Therefore,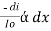 or
or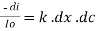 Integrating within limits Ln
Integrating within limits Ln Or
Or
|
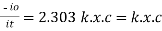 Therefore, K is absorptivity constantThe term log
Therefore, K is absorptivity constantThe term log 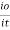 is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution (
is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution ( ) and unit length of path (
) and unit length of path (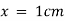 ) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
|
|
|
UNIT – II INSTRUMENTAL METHODS OF CHEMICAL ANALYSIS Q1) What is the advantage of instrumental method?The minute sample quantity is required. The instrumental method determination is considerably fast. Complex mixture can be analysed either with or without their separation. Sufficient reliability and accuracy of results are obtained by instrumental method. When non-instrumental method is not possible, instrumental method is the only answer to the problem. Q2) What is the disadvantage of instrumental method?A2)Instrumental methods are costly because of cost, maintenance and trained personnel required for their handling. The used instrument defines sensitivity and accuracy. Specialized training for handling instrument is required. There is frequent need of checking results with other methods. In some cases, instrumental method may not be specific. Q3) What is spectrometry?A3) A spectrometer is used to measure wavelengths of electromagnetic radiation that has interacted with a sample. Incident light can be reflected off, absorbed by, or transmitted through a sample. The way the incident light changes during the interaction with the sample is characteristic of the sample. A spectrometer measures this change over a range of incident wavelengths.
There are three main components in all spectrometers; these components can vary widely between instruments for specific applications and levels of resolution. Generally, these components produce the electromagnetic radiation, somehow narrows the electromagnetic radiation to a specified range, and then detect the resulting electromagnetic radiation after is has interacted with the sample. Q4) What is electromagnetic radiation?A4) Electromagnetic radiations are a form of energy that is transmitted through space with high velocity. Electromagnetic radiations consist of discrete packets of energy which are called photons. A photon consists of an oscillating electric field (E) and an oscillating magnetic field (M). Q5) Explain Lambert’s law.A5) The rate of decrease in intensity of radiation is directly proportional to path length of solution when the monochromatic light passes through a solution of constant concentration.Mathematically the law can be written as,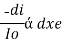 Therefore,
Therefore, intensity (radiant power) of the incident radiation.
intensity (radiant power) of the incident radiation.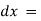 small thickness of solution or path length.
small thickness of solution or path length.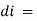 small decrease in intensity of light
small decrease in intensity of light  Intensity or radiant power is the number of photons per unit area per second
Intensity or radiant power is the number of photons per unit area per second 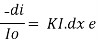 Integrating within limits
Integrating within limits 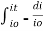 =
= 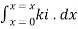 Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be
Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be 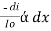 -- --------------------- (lamberts law for constant concentration)
-- --------------------- (lamberts law for constant concentration) ----------------------- - - (beers law for constant thickness of solution)Therefore,
----------------------- - - (beers law for constant thickness of solution)Therefore,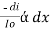 or
or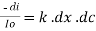 Integrating within limits Ln
Integrating within limits Ln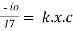 Or
Or
Log 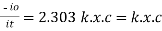 Therefore, K is absorptivity constantThe term log
Therefore, K is absorptivity constantThe term log 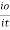 is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution (
is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution (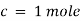 ) and unit length of path (
) and unit length of path (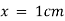 ) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
Q8) What is Adsorption chromatography?A8) This chromatography is one of the oldest type of chromatography. It utilizes a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibrium between the mobile and stationary phase accounts for the separation of different solutes.
Q9) Explain gas liquid chromatograph.A9) This is the one of the fastest separation method, which allows for the quantitative determination of each compound of the mixture. In the Gas-Liquid chromatography the process begins by the bringing of sample by the syringe to the heated injection chamber. The sample is passing through the nitrogen gas in the column. The same column is has the thin film of liquid. After the separation of the carried gas the separated mixture flows in detector which activates a recorder. Traces a series of peaks on chromatogram.
Q10) What are the applications of chromatography?A10)1) The chromatographic technique is used for the separation of amino acids, proteins and carbohydrates.2) It is helpful for the qualitative and quantitative analysis of complex mixtures.3) It is also used for the analysis of drugs, hormones, vitamins and brain amines.4) This technique is also helpful for the determination of molecular weight of proteins.5) It is helpful in detection of phenolic compounds in drinking water.6) It is helpful in detection of endogenous Neuro peptides in extracellular fluid of brain etc.
A1) Advantages of instrumental methods:-
|
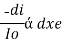 Therefore,
Therefore, intensity (radiant power) of the incident radiation.
intensity (radiant power) of the incident radiation.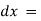 small thickness of solution or path length.
small thickness of solution or path length.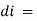 small decrease in intensity of light
small decrease in intensity of light  Intensity or radiant power is the number of photons per unit area per second
Intensity or radiant power is the number of photons per unit area per second 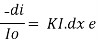 Integrating within limits
Integrating within limits 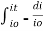 =
= 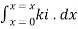 Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be
Therefore,(ln it – ln io) = ki. xIn io/it = ki.xLambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration. Q6) Explain Lambert-Beer law.A6) The combined lamberts – beers law will be 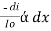 -- --------------------- (lamberts law for constant concentration)
-- --------------------- (lamberts law for constant concentration) ----------------------- - - (beers law for constant thickness of solution)Therefore,
----------------------- - - (beers law for constant thickness of solution)Therefore,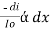 or
or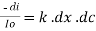 Integrating within limits Ln
Integrating within limits Ln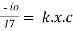 Or
Or
|
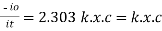 Therefore, K is absorptivity constantThe term log
Therefore, K is absorptivity constantThe term log 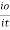 is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution (
is defined as absorbance (a) and transmittance (T) is defined as it/ioA= K.X.C Lambert – beers lawTherefore,X is in om C is in gm/lit Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.By taking unit concentration of sample in solution (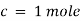 ) and unit length of path (
) and unit length of path (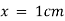 ) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.A= E.X.C Therefore, E is known as molar absorptive or molar extinction coefficient. Q7) Explain thin-layer chromatography.A7) The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
|
|
|
0 matching results found