Question Bank Module 06
Q-Explain impurities in water.
Hardness can be defined as a soap consuming capacity of water sample. Soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. They dissolve readily .in water to form lather due to which it has cleansing property.


- But compounds of fatty acids with other metals done dissolve in water.
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd.
 2
2
(calciumstearate)
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd
- These other metal ions are responsible for the hardness of water most important metal of ions which cause hardness to water are calcium and magnesium ions.
- The hardness of water along can be calculated from the amount of calcium and magnesium ions present in water along with bicarbonates, sulphates chlorides and nitrates.
Q-Explain Hardness of Water.
1. Parts Per Million (ppm):- is the parts of CaCO3 equivalent hardness per 106 parts of water i.e., 1ppm= 1 part of CaCO3 equivalent hardness in 106 parts of water.
2. Milligrams Per Litre (mg/L):- number of milligrams of CaCO3 equivalent hardness present per liter of water.
1mg/L=1mg of CaCO3
Equivalent hardness of 1L of water= 1kg=1000g=106mg.
∴1mg/L=1mg of CaCO3 eq per 106 mg of water=1ppm.
3. Clarke’s degree (0Cl):- the no. Of grains (1/7000lb) of CaCO3 equivalent hardness per gallon (10lb) of water or it is parts of CaCO3 equivalent hardness per 70,000 parts of water.
∴1 0Cl= 1 grain of CaCO3 eq hardness per gallon of water
= 1 part of CaCO3 hardness eq per 105 parts of water.
4. Degree French ( o Fr):- parts of CaCO3 equivalent hardness per 105 parts of water.
∴1 0 Fr= 1 part of CaCO3 equivalent hardness per 105 parts of water.
5. Milli-equivalent per liter (meq/L):- is the number of milli-equivalents of hardness present per liter.
1meq/L = 1meq of CaCO3 per liter of water
= 10-3 x 50 g of CaCO3 eq. Per liter
= 50 mg of CaCO3 eq. Per liter
= 50 mg/L of CaCO3 eq.
= 50ppm.
Q-Explain different type of Hardness.
- Temporary hardness ( carbonate) :-
- When water containing calcium and magnesium bicarbonates is heated , bicarbonate decompose and from insoluble carbonate and form hydroxide.
- On filtering such water , soft water is obtained.
- The hardness which can be removed by more boiling is referred as ‘temporary hardness ’ or bicarbonate hardness.

 Ca
Ca

Mg 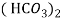
 Mg
Mg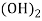 + 2 CO
+ 2 CO 
(Bicarbonates)
II. Permanent hardness :-
- The term permanent hardness ornon carbonate is the term applied to the hardness caused by dissolved chlorides , nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
III. Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate , calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
4. As the sulphate and chloride are neutral salts , the hardness caused by presence of calcium and magnesium sulphate , chlorides and nitrates is termed as non alkaline hardness or non carbonate hardness.
IV. Total hardness :-
The hardness due to all hardness causing salts , known as total hardness.
Total hardness = temporary + permanent
Q-Explain the titration method of EDTA.
Titration with EDTA
EDTA(Ethylene Diamine Tetra Acetic Acid) , has four carboxyl groups and two amine groups that can act as electron pair donors. The ability of EDTA to potentially donate its six lone pairs of electrons for the formation of coordinate covalent bonds to metal cations makes EDTA a hexadentate ligand. However, in practice EDTA is usually only partially ionized, and thus forms fewer than six coordinate covalent bonds with metal cations.
Disodium EDTA is commonly used to standardize aqueous solutions of transition metal cations. Disodium EDTA (often written as Na2H2Y) only forms four coordinate covalent bonds to metal cations at pH values ≤ 12. In this pH range, the amine groups remain protonated and thus unable to donate electrons to the formation of coordinate covalent bonds. Note that the shorthand form Na4−xHxY can be used to represent any species of EDTA, with x designating the number of acidic protons bonded to the EDTA molecule.
EDTA forms an octahedral complex with most 2+ metal cations, M2+, in aqueous solution. The main reason that EDTA is used so extensively in the standardization of metal cation solutions is that the formation constant for most metal cation-EDTA complexes is very high, meaning that the equilibrium for the reaction:
M2+ + H4Y → MH2Y + 2H+
Lies far to the right. Carrying out the reaction in a basic buffer solution removes H+ as it is formed, which also favors the formation of the EDTA-metal cation complex reaction product. For most purposes it can be considered that the formation of the metal cation-EDTA complex goes to completion, and this is chiefly why EDTA is used in titrations and standardizations of this type.
Q-Explain the determination of hardness of water by EDTA method.
Principle: This is a complex metric method. It is in the form of its sodium salt which yields the anion and this forms complex with Ca+2 and Mg+2 ions.
(Molecular Wt. - 372.24, Equivalent Wt. - 186.14 i.e., M=2N)

In order to determine the equivalence point (i.e., just completion of metal-EDTA complex formation) indicator Eriochrome Black-T (EBT) an alcoholic solution of blue dye is employed which forms an unstable wine red complex with Ca+2 and Mg+2 ions. The indicator is effective at about pH 10. When EBT is added to hard water, buffered to a pH of about 10 (employing NH4OH-NH4Cl buffer), a wine red unstable complex is formed. Thus,
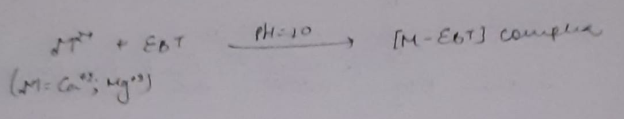
During the course of titration against EDTA solution, EDTA combines with M+2 (or Ca+2 or Mg+2) ions from stable complex M-EDTA and releasing free EBT, which instantaneously combines with M+2 ions still present in the solution, thereby wine red color is retained. Thus, titration

When nearly all M+2 (Ca+2 or Mg+2) ions have formed [M-EDTA] complex, then next drop of EDTA added drop wise displace the EBT indicator from [M-EBT] complex and wine red color changes to blue color (due to EBT). Thus at equivalence point

Q- What are the steps involved in the determination of hardness of water by EDTA.
Steps involved:
1. Preparation of Standard Hard Water: Dissolve 1gm of pure dry CaCO3 in minimum quantity of dil. HCl and then evaporate the solution to dryness on water bath. Dissolve the residue n distilled water to make 1L solution. Each 1ml of this solution contains 1mg of CaCO3 hardness. 6
2. Standardization of EDTA solution: Rinse and fill the burette with EDTA solution. Pipette out 50ml of standard hard water in a conical flask. Add 10-15ml of buffer solution and 4 drops of indicator. Titrate with EDTA solution till wine red color changes to clear blue. Let the volume used be V1ml.
3. Titration of Unknown Hard Water: Titrate 50ml of water sample just in step5. Let the volume used be V2ml.
4. Titration of Permanent Hardness: Take 250ml of water sample in a large beaker. Boil till the volume is reduced to about 50ml (all the bicarbonates are decomposed into insoluble CaCO3+Mg(OH)2). Filter, wash the precipitate with distilled water collecting filtrate and washings in a 250 ml measuring flask. Finally make up the volume to 250ml with distilled water.
Q- Explain Ion Exchange method.
Ion exchange or de ionization or de mineralization ion exchange resins are insoluble cross linked long chain organic polymers with a microporous structure and the functional group attached to the chains are responsible for the ion exchange properties resins contaning acidic function groups are capable of exchaning their anions with other anions which comes in their contact the ion exchange resins may be classified as :-
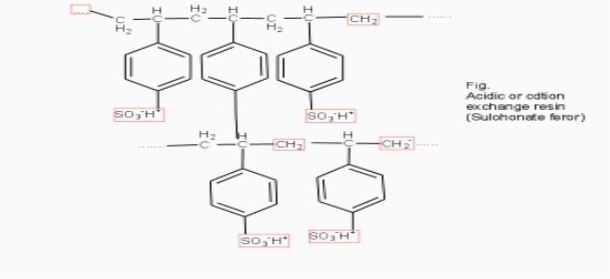
Cation exchange resins ( RH+) :-
They are mainly styrene divinly benzene co-polymers which on sulphonation or carbonoxylation become capable to exchange their hydrogen ions with the cations in the water.
- Anion exchange resins :-
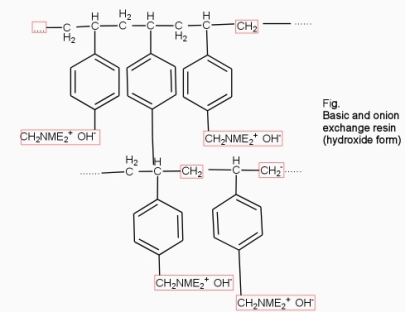
They are styrene – divinly benzene or amine fermaldehyde which contain amino or quaternary ammonium or4 quaternary phosphonium or tertiary sulphonium group as an integral part of the resin matrix.these after treatment with dilute NaOH solution become capable to exchange their OH- anions with anions in water .
Processes :-
The hard water is passed first through cation exchange column which removes all the cations like Ca²+ , Mg² + e.t.c . From it and equivalent amount of H+ ions are released from this column Water
2RH+ + Ca².+ ---------------------- R2Ca2+ + 2H
2RH+ Mg2+ --------------------------- R2Mg2+ 2H+
After cation exchange column the hard water passed through anion like cl- . Present in the water and equivalent amount of OH- ions are released from this column to water,
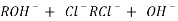

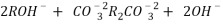
H+ And OH – ions ( released from cation exchange and anion exchange columns respectively ) get combined to produce water molecule.
H+ + OH -  H2O
H2O
Thus,
The water coming out form the exchanger is free from cations as well as an ions . Ion free water is known as de mineralizes water.
Regeneration :-
When capacities of cation and anion exchangers to exchange H+ and OH- ions respectively . Are lost they are then said to be exhausted.
The exhausted cation exchange column is regenerated by passing a solution of dil.HclH2SO4. The regeneration can be represented as
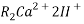

 ( washing )
( washing )
The column is washed with deionized water and washing ( which contains Ca2+ and Cl2- ions ) is passed to sink or drain.
The exhausted anion exchange column is regenerated by passing a solution of dil. NaOH.
The column is washed with de-ionized water and washing (which contains NA+ and 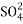 ions ) is pass to sink or drain.
ions ) is pass to sink or drain.
The regenerated ion exchange ion exchange resins are then used again.
Q- What are the advantage and disadvantage of ion exchange method.
Advantages :-
- The processes can be used to soften highly acidic or alkaline waters.
- It produces water of very low hardness ( say 2 ppm ) so it is very good for treating water for use in high pressure boilers.
Disadvantages :-
- The equipment is costly and more expensive chemicals are needed.
- If water contains turbidity, then the out- put of the processes is reduced .the turbidity must be below 10 ppm if it is more it has to be removed first by filtration.
Q-Explain BOD.
The determination of the Biochemical Oxygen Demand or Biological Oxygen Demand (BOD) evaluates the amount of biodegradable organic material present in wastewater, effluent and polluted waters. The BOD test reflects the amount of dissolved oxygen (DO) consumed by bacteria while oxidizing these materials. Dissolved oxygen is essential for the life of aquatic fauna and flora, and the BOD test is a measure of the ecological impact that effluent water may have on the receiving body of water (river, lake, etc.). This test is often required in discharge permits, as it is a means of assessing the degree of water pollution.
Q- Explain COD.
The chemical oxygen demand (COD) is a measure of water and wastewater quality. The COD test is often used to monitor water treatment plant efficiency. This test is based on the fact that a strong oxidizing agent, under acidic conditions, can fully oxidize almost any organic compound to carbon dioxide. The COD is the amount of oxygen consumed to chemically oxidize organic water contaminants to inorganic end products.
The COD is often measured using a strong oxidant (e.g. Potassium dichromate, potassium iodate, potassium permanganate) under acidic conditions. A known excess amount of the oxidant is added to the sample. Once oxidation is complete, the concentration of organics in the sample is calculated by measuring the amount of oxidant remaining in the solution. This is usually done by titration, using an indicator solution. COD is expressed in mg/L, which indicates the mass of oxygen consumed per liter of solution.
COD is a second method of estimating how much oxygen would be depleted from a body of receiving water as a result of bacterial action. The COD test uses a strong chemical oxidizing agent (potassium dichromate or potassium permanganate) to chemically oxidize the organic material in the sample of wastewater under conditions of heat and strong acid. The COD test has the advantage of not being subject to interference from toxic materials, as well as requiring only two or three hours for test completion, as opposed to five days for the BOD test. It has the disadvantage of being completely artificial, but is nevertheless considered to yield a result that may be used as the basis upon which to calculate a reasonably accurate and reproducible estimate of the oxygen-demanding properties of a wastewater. The COD test is often used in conjunction with the BOD test to estimate the amount of non-biodegradable organic material in a wastewater. In the case of biodegradable organics, the COD is normally in the range of 1.3 to 1.5 times the BOD. When the result of a COD test is more than twice that of the BOD test, there is good reason to suspect that a significant portion of the organic material in the sample is not biodegradable by ordinary microorganisms. As a side note, it is important to be aware that the sample vial resulting from a COD test can contain leachable mercury above regulatory limits. If such is the case, the sample must be managed as a toxic hazardous waste.
Q-What are the applications of Electro-dialysis.
Applications:
- Large scale brackish and seawater desalination and salt production.
- Small and medium scale drinking water production (e.g., towns & villages, construction & military camps, nitrate reduction, hotels & hospitals)
- Water reuse
- Pre-demineralization
- Food processing
- Agricultural water
- Glycol desalting
- Glycerin purification
Q- Explain Reverse Osmosis.
Reverse osmosis is a process where the water is separated from the salts in the source water by pressure-driven transport through a membrane. This process uses semi-permeable membrane and applied pressure to preferentially induce water permeation through the membrane while rejecting salts. The RO plant uses less energy than thermal desalination process. This process uses thin-film composite membrane that too comprises of ultra thin aromatic polyamide thin film. The used polyamide film gives the transparent properties while the remaining part provides the mechanical supports. The polyamide films are very dense void free polymer with high surface area allowing for its high permeability. RO desalination plant include source water intake system, pretreatment facilities and high pressure feed pumps, RO membrane trains, energy recovery and desalinated water conditioning system. The intake system may be the open surface water intake or series of seawater beach wells. The pretreatment system may be the screening, chemical conditioning, sedimentation or filtration that totally depends on the used quality water further the filtered water is conveyed by transfer pump from filtrate water storage tank through cartridge filter and into the suction pipe of high pressure RO feed pumps. The cartridge filters are designed in such a manner that can retain 1 to 20 microns particles which remained in the source water after pretreatment. The high pressure feed pumps are designed to deliver the source water to the RO membranes at pressure required for membrane separation of the fresh water from the salts. The actual required feed pressure is site-specific and is mainly determined by the source water salinity and the configuration of the RO system.